Isotopes of an element.
science·@ahmadchemist737·
0.000 HBDIsotopes of an element.
Hy!
Steemit friends!
Today I am describing the important topic chemistry,Isotopes of element.
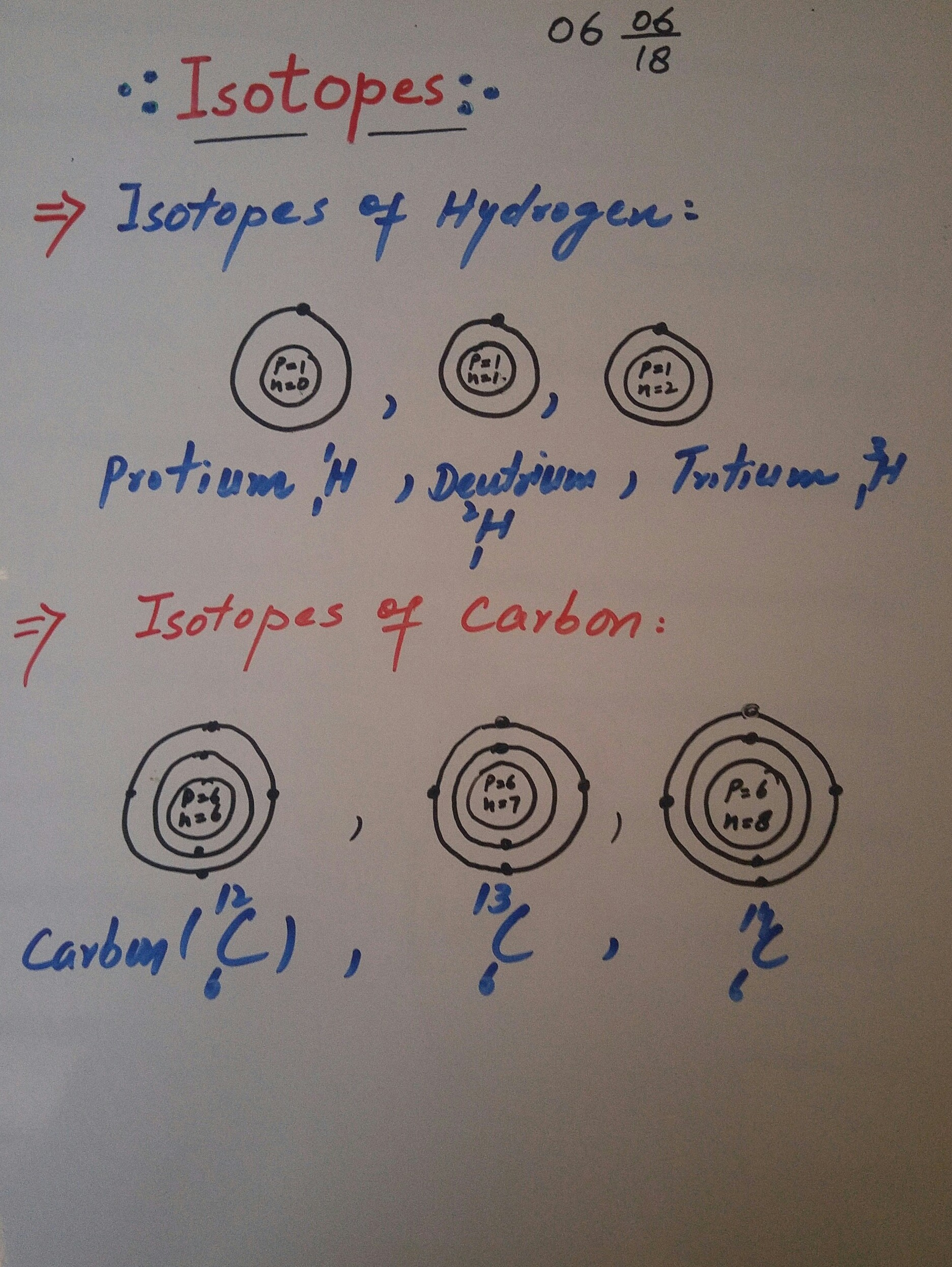
ISOTOPE:
The atoms of an element having the same atomic number but different mass number are called isotopes of that element.
Since atomic number represent no of proton and mass number is sum of proton and neutron.So the isotope have same number of proton but neutron number.
The chemical properties depend upon atomic number will be same and mass and physical properties depend upon mass number will be different.
Examples of istopes are explained in diagram.
👍 beetlevc, fonteynb, otherglens, dick.sledge, msp-bidbot, honestbot, isotonic, lost-ninja, sr-adnan, ahmadchemist737, moneymatchgaming, bid4joy, shares, adriatik, seakraken, th3voter, cesursincap, peoplesbot, aksdwi, sunrawhale, whalepromobot, joeparys, whalecreator, booster, arcange, raphaelle, bodzila, dolphinbot, dailyupvotes, siditech, peace-bot, ubot, upyourpost, postpromoter, sureshot, mercurybot, estabond,