Materials Science I: Polymers
education·@bape682·
0.000 HBDMaterials Science I: Polymers
><center>Hi Steemit, I want to start doing a series of topics in the area of materials science, I will start in this Post with the polymers, I hope you like it.</center> <center></center> ## <center>***Polymers***</center> <center>The first thing to know is that the term polymer is derived from the Greek words poly and mere, which mean many parts. In this sense, the polymers are chemical species with high molar masses, formed by the sequential or random union of molecules of smaller mass called monomers, by means of covalent bonds. In our bodies, as in all life forms, these macromolecules are present in the form of carbohydrates, proteins, nucleic acids, etc., polymeric materials such as wood, natural resins, gums and fibers such as cotton, wool, silk and so on, have been used to satisfy human needs since ancient times.</center> <center>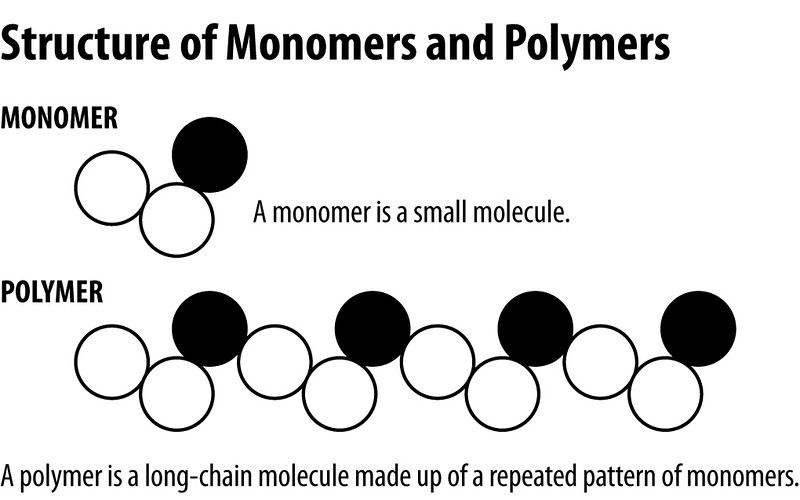</center><center> <a href="https://photos.smugmug.com/Graphics/Graphics/i-w9NJQXC/1/ed40b27d/L/structure%20of%20monomer%20and%20polymer-L.jpg">Polymers</a> </center> ## <center>***Classification of polymers***</center> <center>There are several methods for classifying polymers. According to their response to the heat treatment applied to them, they are divided into thermoplastics and thermosets. Thermoplastics are polymers that melt when heated and resolidify when cooled, while thermosets are those that do not melt when heated, but at sufficiently high temperatures, they decompose irreversibly. Other classes of polymers are also the elastomers and the fibers</center> <center>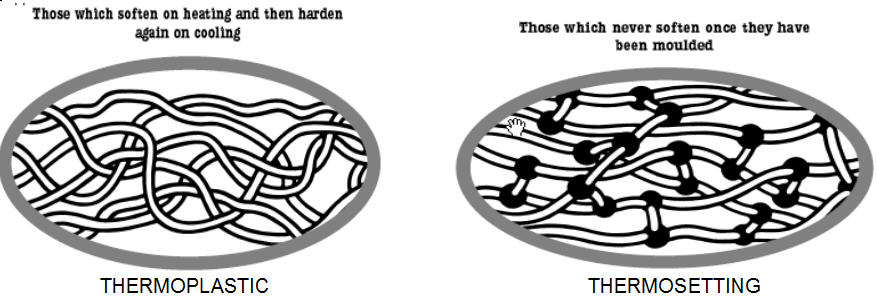</center><center> <a href="http://www.ruthtrumpold.id.au/destech/?page_id=83">Thermoplastics and thermosets</a> </center> <center>Another classification system is based on the nature of the chemical reactions employed in the polymerization and may be condensation polymers prepared from monomers where the reaction is accompanied by the loss of a small molecule, usually water, or addition polymers formed by the addition reaction of an unsaturated monomer.</center> ## <center>***Physico-chemical characteristics***</center> <center>The physico-chemical characteristics of these polymers are determined by the design achieved by the polymerization reactions, where the polymer chains formed from a single type of monomer are called homopolymers, whereas those formed from more than one unit are defined as copolymers. Depending on the shape of the chain, the homopolymers may be linear, branched or crosslinked. In the copolymers, the monomers can be ordered in the main chain alternately, randomly, block or functionalized.</center> <center>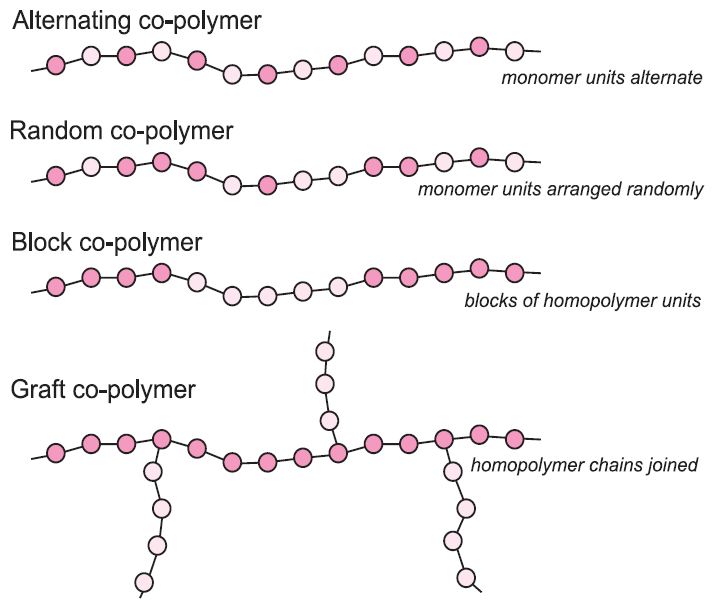</center><center> <a href="https://www.google.co.ve/imgres?imgurl=http%3A%2F%2Fwww.essentialchemicalindustry.org%2Fimages%2Fstories%2F530_Polymers%2FPolymers_33.JPG&imgrefurl=http%3A%2F%2Fwww.essentialchemicalindustry.org%2Fpolymers%2Fpolymers-an-overview.html&docid=soZEd3hyhvMJcM&tbnid=P6DTBLAN9X-o1M%3A&vet=10ahUKEwiiu7Lbj7LWAhXslVQKHSoyA7Y4ZBAzCAkoBzAH..i&w=713&h=613&bih=541&biw=1215&q=polymers&ved=0ahUKEwiiu7Lbj7LWAhXslVQKHSoyA7Y4ZBAzCAkoBzAH&iact=mrc&uact=8">Physico-chemical characteristics</a> </center> <center>The properties mentioned above give the polymeric materials a high resistance to the environments to which they are subjected. So they can have multiple applications, facilitated our lives and being one of the engines of the development of materials science. However, the high resistance of these materials generates an environmental problem, which is currently the subject of a large amount of research, mainly with biodegradable polymers, which will expand in another Post.</center> <center>If you liked the post and want to continue seeing more articles of this type, upvote, follow me and resteem.</center> >References: >Akkapeddi, K. 2.001. Rubber toughening of polyamides by reactive blending. En: Reactive Polymer Blending. Baker W., Scott C., Hu G.-H., Hanser (eds). Munich. Págs. 207-208. >Martinelli, M.; Froimowicz, P.; Calderon, M. y Strumia, M. 2003. Materiales poliméricos funcionalizados. Parte I: síntesis y polimerización de monómeros funcionalizados. Revista Iberoamericana de Polímeros, 04: 30-46.
👍 bape682, daniel82, fredrikaa, coinbitgold, justtryme90, steemstem, foundation, timothyb, kenchung, dber, kharrazi, timsaid, dna-replication, jamhuery, mobbs, professorbromide, ovij, lemouth, curie, alexander.alexis, velourex, lamouthe, garudi, anwenbaumeister, hendrikdegrote, the-devil, kyriacos, kushed, abigail-dantes, cotidiana, ch00fy, ananiani, pharesim, randyclemens, cheah, shahzadnisar, n1kofi, ben.zimmerman, sellergenius, beeskee, marcosespes1, shehbaznawaz, raymondspeaks, abh12345, ninkhisibir, ourlifestory, toxichan, mrlogic, sethlinson, steemedia, cebymaster, allgoodthings, krizia, blackwidow7, tabea, john-gpr, bp423, alainite, mxzn, abishai, locikll, palmtreetrading, irwanumpal, cryptoninja, glitterfart, oscar2020, danig2108, asmolokalo, djnoel, revisesociology, cjzachary, juniorfelicio, asunarose, lafona-miner, andrewfilisetti, duekie, mcw, ghasemkiani, zest, aarkay, zacharius, penstand, jhpauque, crypticalias, tanyagry993,