Where Does the Periodic Table End? Super heavy elements.
steemstem·@frankjavier·
0.000 HBDWhere Does the Periodic Table End? Super heavy elements.
> # In chemistry, the transactinidium elements or superheavy elements are those with an atomic number greater than that of the heaviest element in the series of actinides, lawrencio (103). These elements are also transuranic, that is, with an atomic number higher than that of uranium (92), an actinide. <center></center><center>[***Source***](http://physicsworld.com/cws/article/news/2014/may/09/superheavy-element-117-weighs-in-again)</center> # A little history Both the concept of atom and element have their origin in classical Greece. Already then some thinkers believed that matter should be composed of small and indivisible parts, atoms, and different types of bricks since it was clear that water, wood, and fire, for example, had a very different behavior. Therefore, some considered that there were four elements: air, fire, water, and earth. This idea survived in the West for centuries and centuries. The alchemists began to modify it when they began their studies with metals. But with the flowering of chemistry especially in the eighteenth century, this idea died at last: Lavoisier and others introduced the concept of a chemical element, as a material with characteristic properties that could be measured. In fact, Mendeleev, who was not the first to elaborate a table of chemical elements, managed to design a classification based on these chemical properties and that allowed to predict how the elements not yet discovered would be based on them. This creation is known as the periodic table of the elements because it is built on the basis of characteristics that are repeated in a cyclic (periodic) manner. It was then the year 1869 (the American Civil War had ended about four years ago). At that time, the meticulous and constant work of several generations of chemists had managed to discover 63 chemical elements. On December 30, 2015, Mendeleev was again at the front. The International Union of Pure and Applied Chemistry announced the incorporation of four new chemical elements to the periodic table, with which the repertoire of known chemical elements reached the figure of 118. In addition, the seventh row of the table was complete and the time came to open a new row to continue discovering elements. # Superheavy elements The discoveries of elements 113, 115, 117 and 118 were officially confirmed by the International Union of Pure and Applied Chemistry. The group has the task of examining the elements produced by a man who seeks a permanent place in the iconic table that adorns chemistry classrooms around the world. The new elements are known as superheavy elements because the nuclei of their atoms are huge. Element 118, for example, is the heaviest element to date, with 118 protons along with 176 neutrons. Elements of this size are not usually found in nature, and it can take years to produce them in specialized laboratories. > # "Probably the only other place where they could exist in a short period of time could be in a supernova, where there are so much energy and so many particles that are really very concentrated," said Dan Shaughnessy, principal investigator for the Heavy Elements Group of the Lawrence Livermore National Laboratory, which contributed to three of the discoveries. The superheavy elements are also highly unstable, existing for a fraction of a second before they begin to deteriorate. Scientists never observe these elements directly. On the contrary, they know that they existed briefly because they are able to measure their disintegration products. The heaviest known elements are created by crashing one particle against another, hoping that they will stick together. It's a probability game with extremely long possibilities. Scientists first create an objective from a careful selection of an atom with a certain number of protons and neutrons - a process that can last for months. Then they purify it and bombard it with another specialized atom that they believe has a better chance of recombining with the target. <center>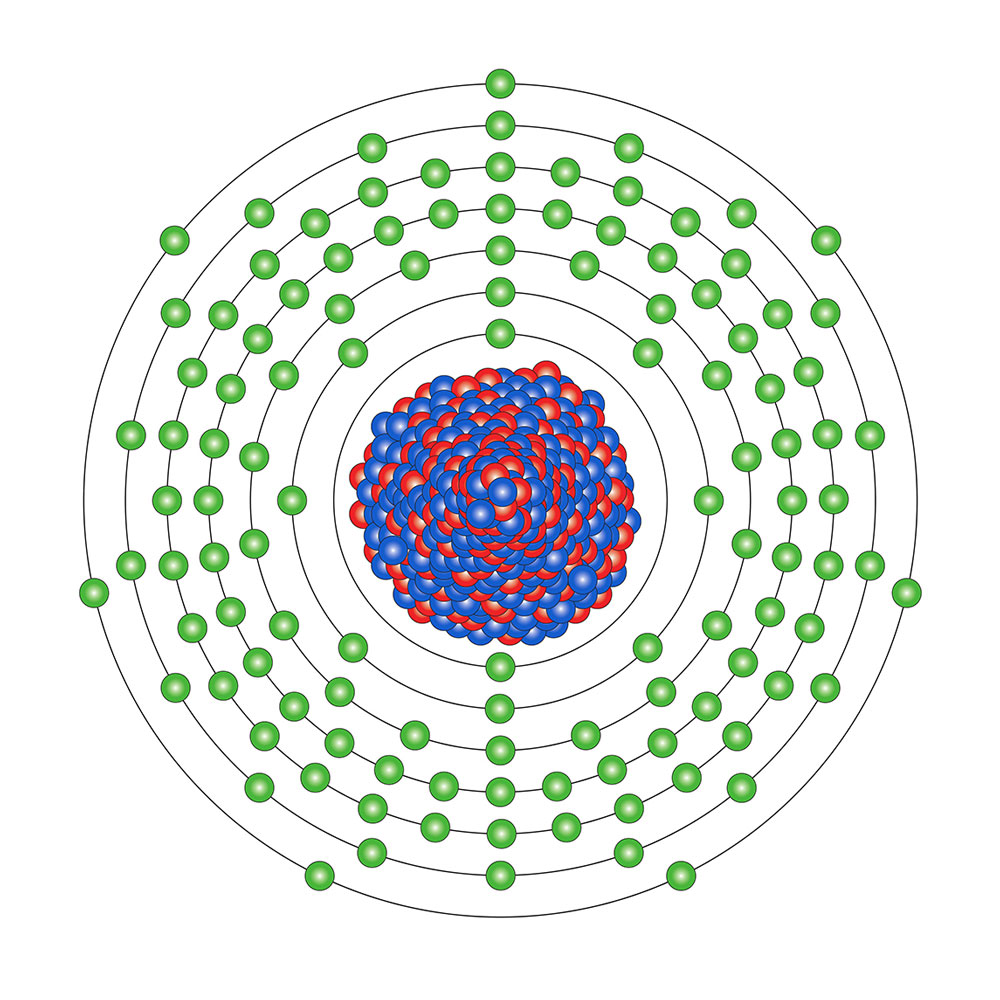</center><center>[***This diagram of element 117, the most recently discovered member of the periodic table, shows its 117 protons (red) and 117 electrons (green). Shown here is the most stable isotope of the element, ununseptium-294, with 177 neutrons (blue) Source***](http://discovermagazine.com/2015/march/11-forging-new-elements)</center> # Reference ### http://physicsworld.com/cws/article/news/2014/may/09/superheavy-element-117-weighs-in-again ### http://discovermagazine.com/2015/march/11-forging-new-elements ### https://newatlas.com/superheavy-element-117-discovered/14795/ ### http://physicsworld.com/cws/article/news/2006/oct/19/researchers-discover-element-118