Thermodynamic Cycles. Part 1
steemstem·@martinezkarla·
0.000 HBDThermodynamic Cycles. Part 1
The devices that are used to convert heat transfer into work they are called thermal machines. In previous posts when you mention the second law of thermodynamics explain a little of one of the most used cycles ***The Carnot*** cycle to demonstrate the limitations imposed by the second law on the efficiency of all the cycles of the thermal machines that operate between the same temperatures for the addition and rejection of heat. While the Carnet cycle is useful for determining the ideal behavior is not a practical cycle that suits the design of a thermal machine. It is not possible to design a team that allows the transfer of heat to a working fluid at constant temperature, in a reversible process, during a finite time; to achieve this a temperature difference would be necessary infinitesimally small between the system and the surroundings, but with the effect unfortunate that the transfer of energy would require an extremely time big. When you want to operate the Carnot machine with a power level reasonable, it is necessary to carry out many cycles per unit of time and, if this is done, the operation of the machine is very far from the reversible ideal case. The same difficulty arises in the process of heat rejection, which for the true Carnot cycle must also be isothermal and reversible. <center>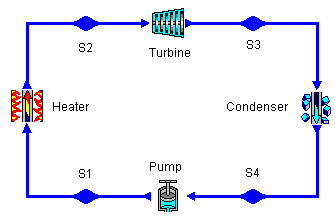</center> <center>[Source](http://www.qrg.northwestern.edu/projects/NSF/Cyclepad/cpadw004.htm)</center> Due to the difficulties mentioned above where an idea cycle is impossible to perform, other cycles have been developed that they have theoretical efficiencies equal to or less than those of the Carnot cycle. The transference of heat in these cycles is generally not isothermal but, when performed in practical machines, their real thermodynamic efficiencies are greater than those obtained in machines with almost isothermal heat transfers. There are other reasons to develop thermal machines that do not operate with the Carnet cycle. These include the characteristics of the energy sources available to drive the thermal machine, the characteristics of the working fluid selected for the cycle, the material limitations of the equipment, the requirements for stable or variable work delivery and other practical considerations. By these reasons and given the difficulty of building an efficient thermal machine that actually operates almost with a Carnot cycle, have been proposed throughout the various different cycles for the thermal machines and have built the apparatuses that operate under those cycles. <center>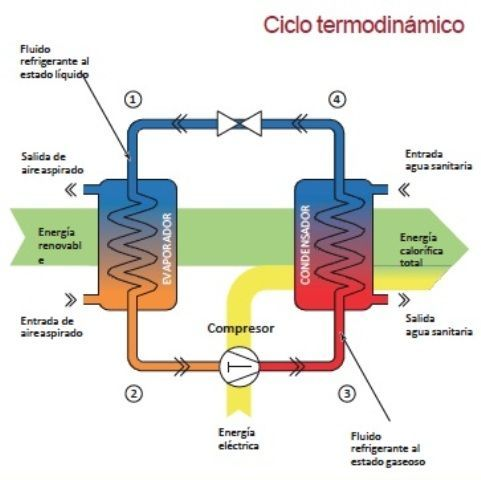</center> <center>[Source](http://www.sierterm.es/tiki-index.php?page=ciclo+termodin%C3%A1mico)</center> ### The cycles of the thermal machines are classified in different ways but the most agreed by experts in thermodynamics are the following * Cycles where the energy source is outside the working fluid and the transfer of heat takes place across the borders of the system. Example externa combustion engine <center>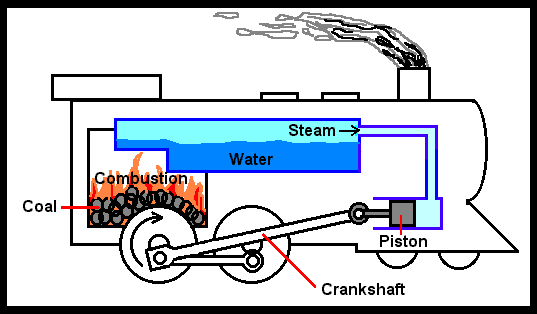</center><center>[Source](http://www.mecanicaymotores.com/el-motor-de-combustion-externa.html)</center> * Machines in which the fuel is burned within the boundaries of the system and the energy thus released is used to increase the temperature of the work fluid. <center>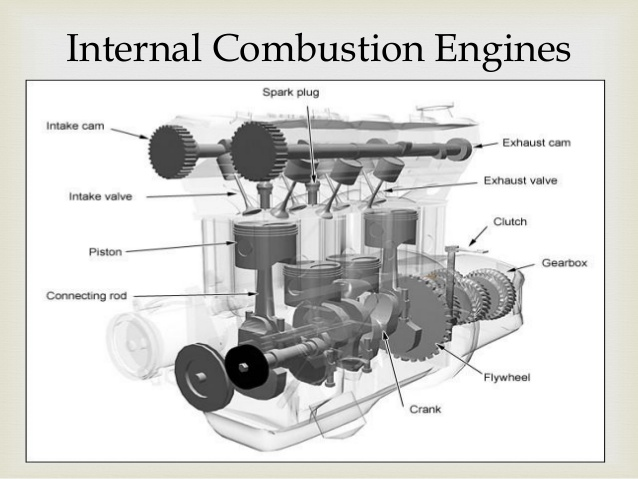</center><center>[Source](https://www.slideshare.net/SLA1987/basics-of-ic-engine)</center> # Cycles with external heat transfer # CARNOT MACHINE. The Carnot cycle (Sadi Carnot, French, 1796 - 1832), is of great importance from the practical point of view as theoretical. Carnot demonstrated that a thermal machine that operated in an ideal reversible cycle between two heat sources would be the most efficient machine possible. An ideal machine of this type, called the Carnot machine, sets an upper limit on the efficiency of all machines. This means that the network done by a work substance carried through a Carnot cycle is the maximum possible for a given amount of heat supplied to the working substance. Carnot's theorem is stated as follows: > No real thermal machine operating between two heat sources, can be more efficient than a Carnot machine, operating between the same two sources. <center>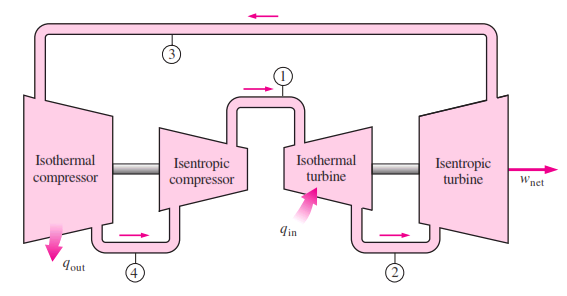</center><center>[Source](http://sounak4u.weebly.com/gas-power-cycle.html)</center> # Cyclo the Stirling The engine operates with an external heat source that can be even solar or nuclear and a heat sink the temperature difference between both sources must be large. In the heat-to-work conversion process, the Stirling engine achieves superior performance to any other real engine, approaching the maximum possible of the ideal Carnot engine. In practice it is limited because the gas with which it works is not ideal, friction is inevitable in the different components that move. The Stirling cycle uses a compressible fluid as a working fluid and consists of a process of energy addition at constant temperature followed by a process at volume constant, then a rejection of energy at constant temperature and, finally, returns to its initial state by another process at constant volume, as seen in the figure. <center>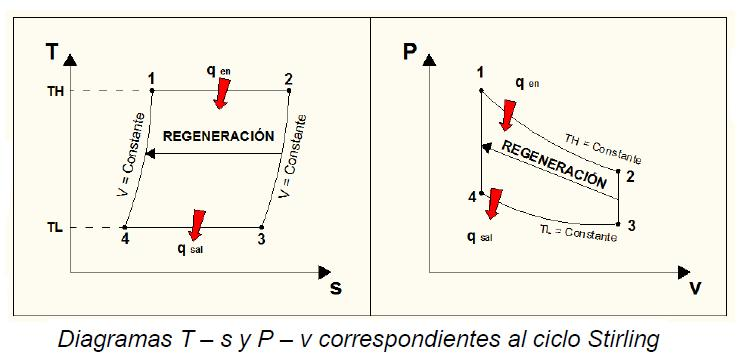</center><center>[Source](https://jmirez.wordpress.com/category/motor-stirling/)</center> An interesting attribute of the Stirling machine is that the working fluid corresponds to a closed system and is always contained within the machine, so that there is no contamination of the lubricant. In addition, the working fluid it is chosen for its thermal properties since the amount needed is very small and the cost is not high. <center>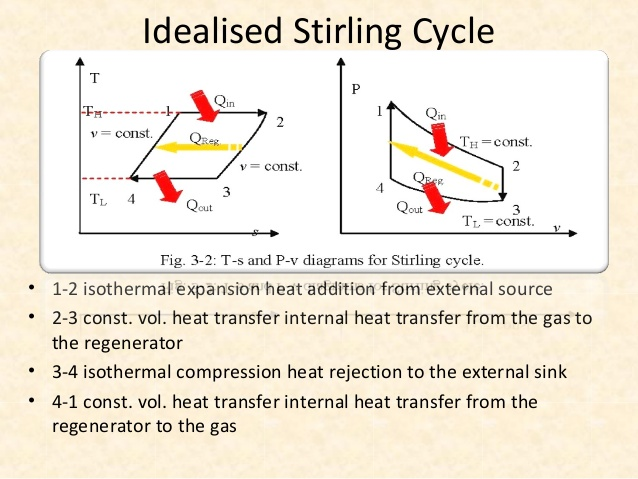</center><center>[Source](https://www.slideshare.net/rajuthegrest/stirling-cycle-its-applications-32767998)</center> # Cycle the Ericsson The Ericsson cycle was conceived by the inventor John Ericsson, who designed and built several hot air motors based on different thermodynamic cycles. Ericsson engines are based on the Ericsson cycle. They are external combustion so the engine gas is heated from the outside. To improve the performance (thermal and total) the Ericsson engine has a regenerator or heat recovery. It can operate in open or closed cycle. Expansion and compression occur simultaneously, on opposite sides of the piston. The Ericsson cycle was originally developed, as was the Stirhng cycle, in an effort to find a machine with practical external heat transfer, which replaced water vapor with air as the working fluid. The cycle consists of two constant pressure processes connected by constant temperature processes, one for intake and the other for energy rejection. Again in the ideal reversible cycle, the efficiency must approach that of the Carnot cycle, since the Ericsson cycle operates between two constant temperatures, to which the energy is added and rejected. <center>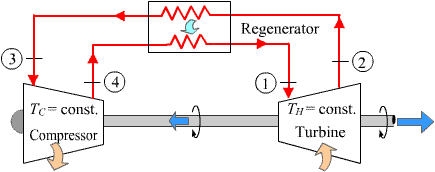</center> <center>[Source](https://yulitiqueesquivel.wordpress.com/ciclos-termodinamicos/)</center> For more information consult the bibliography or links that I leave here: * http://www.nuclear-power.net/nuclear-engineering/thermodynamics/thermodynamic-cycles/ * http://www.unistudyguides.com/wiki/Thermodynamic_Cycles * http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/node23.html * https://jmirez.wordpress.com/category/motor-stirling/ * https://www.slideshare.net/rajuthegrest/stirling-cycle-its-applications-32767998