⚡#FreeEnergy Part 3: Atomic Physics, Properties, Behavior Overview
freeenergy·@mes·
0.000 HBD⚡#FreeEnergy Part 3: Atomic Physics, Properties, Behavior Overview
https://youtu.be/SLW2hcEWTEE
▶️[Watch on 3Speak](https://3speak.tv/watch?v=mes/rjnmpcar) - [Odysee](https://odysee.com/@mes:8/FreeEnergy-Part-3-Atomic-Physics-Properties-Behavior-Overview:5) - [BitChute](https://www.bitchute.com/video/SLW2hcEWTEE/) - [Rumble](https://rumble.com/v4jhu39-freeenergy-part-3-atomic-physics-properties-behavior-overview.html) - [YouTube](https://youtu.be/SLW2hcEWTEE) - [PDF notes](https://1drv.ms/b/s!As32ynv0LoaIh48nlnM9kSmPjk6pdg)
In #FreeEnergy Part 3 I go over another extensive research video and this time review more closely into atomic physics, the properties of atoms, and their behavior in many natural phenomena and applications. I was originally researching into Pons and Fleischmann’s potentially ground breaking Cold Fusion 1989 paper, but I quickly realized that the background information needed to fully understand their experiment, and electrochemistry in general, required that I undergo an extensive research review of many aspects of mainstream science before I can adequately tackle #FreeEnergy suppressed technology and science.
Thus in this video I cover further research into many aspects related to atomic physics and energy in general, and some of those topics covered are listed below:
- Atoms and Atomic Structure
- Chemical Bonds: Covalent, Ionic, and Van der Waals Forces
- Electromagnetic and Nuclear Forces
- Electric Conductors, Insulators, and Polarity
- Cathode Rays, Vacuum Tubes, and Photoelectric Effect
- Thermal Radiation, Incandescence, and more.
- Semiconductors and Superconductors
- Condensed Matter Physics and Solid State Physics
- Alpha Particles, Radioactive Isotopes, Ionizing Radiation
- Hydrogen Isotopes
- Bohr Model, Electric Cloud, Atomic Orbitals
- Intermolecular vs Intramolecular Forces
- Subatomic Particles, Quantum State, Quantum System, and Energy Levels,
- Atomic Spectral/Emission Lines, Absorption Bands, and Valency
- Bose-Einstein Condensate, Quantum Tunneling, and Exotic Atoms
- Universe, Galaxies, Milky Way Galaxy, Solar System, Planets, and Asteroid Belt,
- Muons, Quarks, Leptons, and Particle Decay
- Muon-Catalyzed Fusion and Disinfo Agent Steven E. Jones…
And yes you heard it right, the last part that I cover in this video is Muon-Catalyzed Fusion and how THE Steven E. Jones worked heavily in this topic while working at Los Alamos Laboratory, the same place where the Atomic Bomb was created. That is the same Steven E. Jones behind both the coverup of Cold Fusion in the late 80s early 90s, as well as using the “Thermite” nonsense to coverup 9/11 Directed #FreeEnergy Technology! So it’s fitting that Jones just has to make an appearance…
Anyways, this is another extremely extensive video in which I am self-learning anything and everything that might be necessary to develop #FreeEnergy. In later parts I will be going over many more topics, such as Magnetism and Electricity to name a few, so make sure to follow along because this series will definitely open your understanding of the reality being hidden from us!
**Stay Tuned for #FreeEnergy Part 4…**
---
- Full #FreeEnergy video series: https://mes.fm/freeenergy-playlist
---
### View Video Notes Below!
---
>[Become a MES Super Fan](https://www.youtube.com/channel/UCUUBq1GPBvvGNz7dpgO14Ow/join) - [Donate](https://mes.fm/donate) - [Subscribe via email](http://mes.fm/subscribe) - [MES merchandise](https://mes.fm/store )
>- [MES Links webpage](https://mes.fm/links) - [MES Links Telegram](https://t.me/meslinks) - [MES Truth](https://mes.fm/truth)
>
>**Reuse of my videos:**
>- Feel free to make use of / re-upload / monetize my videos as long as you provide a link to the original video.
>>**Fight back against censorship:**
>- Bookmark sites/channels/accounts and check periodically.
>- Remember to always archive website pages in case they get deleted/changed.
>
>**Recommended Books:** ["Where Did the Towers Go?"](https://mes.fm/judywoodbook) by Dr. Judy Wood
>
>**Join my forums:** [Hive community](https://peakd.com/c/hive-128780) - [Reddit](https://reddit.com/r/AMAZINGMathStuff) - [Discord](https://mes.fm/chatroom)
>
>**Follow along my epic video series:** [MES Science](https://mes.fm/science-playlist) - [MES Experiments](https://peakd.com/mesexperiments/@mes/list) - [Anti-Gravity](https://peakd.com/antigravity/@mes/series) ([MES Duality](https://peakd.com/antigravity/@mes/antigravity-part-6-video-1-objects-in-rotation-defy-mainstream-physics-mes-duality-concept)) - [Free Energy](https://mes.fm/freeenergy-playlist) - [PG](https://peakd.com/pg/@mes/videos)
>
>---
>
>**NOTE 1:** If you don't have time to watch this whole video:
>
>- Skip to the end for Summary and Conclusions (if available)
>- Play this video at a faster speed.
>-- TOP SECRET LIFE HACK: Your brain gets used to faster speed!
>-- [MES tutorial](https://peakd.com/video/@mes/play-videos-at-faster-or-slower-speeds-on-any-website)
>- Download and read video notes.
>- Read notes on the Hive blockchain $HIVE
>- Watch the video in parts.
>-- Timestamps of all parts are in the description.
>
>**Browser extension recommendations:** [Increase video speed](https://mes.fm/videospeed-extension) - [Increase video audio](https://mes.fm/volume-extension) - [Text to speech](https://mes.fm/speech-extension) ([Android app](https://mes.fm/speech-android)) – [Archive webpages](https://chrome.google.com/webstore/detail/archive-page/gcaimhkfmliahedmeklebabdgagipbia)
---
# #FreeEnergy Part 3

This is going to be another epic and extensive research video so #BuckleUp!
**Topics to Cover**
1. Important Note on my Cold Fusion Research
2. Disclaimer on Orwellian Wikipedia
3. Recap on Nuclear Physics
4. Overview on Atomic Physics, Properties, and Behaviors
5. Atoms, Chemical Elements, Chemical Bonds, Molecules
6. Electric Conductors, Electron Holes, Electrodes, Polarity
7. Cathode Rays, Vacuum Tubes, Voltage, Electric Potential, Photoelectric Effect
8. Incandescence, Electroluminescence, Black Body Radiation, Fluorescence
9. Semiconductors, Doping, Insulators, Superconductors, Transistors
10. Condensed Matter Physics, Solid State Physics, Crystals
11. Bohr Model, Hydron: Proton, Deuteron, Triton; Hydrogen Anion
12. Covalent Bonds, Electronegativity, Ionic Compounds
13. Van der Waals Forces, Dipoles, Intermolecular vs. Intramolecular Forces, Adsorption
14. Hydrogen Bonds, Isotopes of Hydrogen: Protium, Deuterium, Tritium
15. Radionuclides, Ionization Radiation
16. Subatomic Particles, Atomic Nucleus, Electron Clouds, Atomic Orbitals, Potential Well
17. Spin and Magnetic Moments
18. Quantum State and Quantum System
19. Energy Levels, Quantum Numbers,
20. Atomic Spectral/Emission Lines, Absorption Bands
21. Valence Electrons, Bonding Behavior
22. Bose-Einstein Condensate, Quantum Tunneling
23. Universe, Observable Universe, Galaxy, Milky Way, Solar System, Sun, Planets, Supernova, Earth
24. Rare and Theoretical Atoms, Exotic Matter, Antimatter, Antiparticles
25. Muons, Quarks, Leptons, Elementary Particles, W and Z Bozons, Truly Neutral Particles
26. Nuclear Transmutation, Feynman Diagram, Muon-Catalyzed Fusion, and as usual Disinfo Agent Steven E. Jones makes an appearance ;)
27. Summary
**Important Note on my Cold Fusion Research**
After going over my nearly 8 hour Nuclear Physics mainstream overview, I started my quest into researching Pons and Fleischmann's original Cold Fusion paper.
But in the research I was preparing, the background information on Electrochemistry was getting way too large, and I didn't want to do another 8 hour video… ;)
But then the background info on Electrochemistry was getting way too large and I realized that I need to learn a lot more about the mainstream atomic/nuclear/energy science before going over Cold Fusion.
Thus I will break down the background information into multiple parts, in order to develop the necessary knowledge to fully understand Pons & Fleischmann's potentially ground breaking work.
In this video, I will revisit Nuclear Physics to develop a better understanding of the atomic and subatomic structure.
Here at MES, there are no shortcuts or promises of grandeur, so if I need to obtain a PhD level of knowledge on multiple branches of science and mathematics in order to produce #FreeEnergy, whose existence is irrefutable, then so be it! #FollowAlongForTheRide
**Disclaimer on Orwellian Wikipedia**
As always, whenever I cite Wikipedia keep in mind that it is mainly to give a mainstream view of events, names, and general topics.
Since I view Wikipedia as an Orwellian government controlled tool to push the "official" or "mainstream" narrative on all topics, if you find something misleading from Wikipedia (and other mainstream sources) please let me know as it could serve as future video material!
**Recall from my Nuclear Physics Overview video:**
Recall from #FreeEnergy Part 2 on some of the aspects of Nuclear Physics that I covered, which are listed below.
**Atoms, Ordinary Matter, Atomic Nucleus, Protons, Neutrons, Electrons, Isotopes
- Atoms are the smallest unit of ordinary matter.
- Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms.
- Plasma is a gaseous mixture of negatively charged electrons and highly charged positive ions which are distinctly separated such that electric fields, currents, and magnetic fields are created; such as lightening.
- Atoms have a nucleus.
- Atoms have one or more electrons bound to the nucleus.
- Nucleus is made of one or more protons and similar number of neutrons.
- Protons and neutrons are called nucleons.
- 99.94% of an atom's mass is in the nucleus.
- Protons have a positive electric charge.
- Electrons have a negative electric charge.
- Neutrons have no electric charge.
- If number of protons and electrons are equal, that atom is electrically neutral.
- Non-neutral atoms have an overall negative or positive charge, and are called ions.
- An atom, or molecule, with a net positive charge is a cation.
- An atom, or molecule, with a net negative charge is an anion.
- Because of their opposite electric charges, cations and anions attract each other and readily form ionic compounds, such as salts.
- Electrons attracted to nucleus by the electromagnetic force.
- Protons and neutrons are attracted to each other by the strong nuclear force.
- Number of protons defines chemical element of the atom.
- Number of neutrons defines the isotope of the element.
- Number of electrons defines magnetic properties of atoms.
- Chemical bonds between atoms forms molecules.
- Branch of physics studying the Atomic Nucleus is known as Nuclear Physics.
**Quantization, Quantum Mechanics, Particle-Wave Duality, Quantum Tunneling**
- Energy, Momentum, and other quantities are restricted to certain discrete values, such as the charge of an electron, and this referred to as quantization.
- Quantum objects behave both as particles and waves.
- The act of observing affects the observations themselves.
- Physical properties of quantum objects are based on probability rather than finite measurements.
- Quantum Tunneling is the term used to describe the phenomenon of particles seemingly moving past a barrier that classical physics can't explain.
**Fundamental Forces: Gravity, Electromagnetism, Strong, Weak**
- There are 4 known fundamental forces or interactions that appear to be irreducible: Gravitational, Electromagnetic, Strong, and Weak Forces.
- Strong and Weak Forces or Interactions are also known as Strong Nuclear and Weak Nuclear Forces or Interactions.
- It is theorized that all forces are related in one "Theory of Everything".
- Gravity is a force by which all objects with mass (and hence energy) move towards each other.
- Gravity is best described by curvature of spacetime due to uneven mass distribution.
- Black holes are extreme cases of this curvature in which nothing can escape its horizon, including light.
- The Electromagnetic Force is carried by the photon and occurs between electrically charged particles.
- Electricity and magnetism are different manifestations of the same interaction.
- The Strong Force is carried by gluons to bind quarks together to form hadrons such as protons and neutrons.
- The Strong Force has a residual Nuclear Force that binds the hadrons to form atomic nuclei.
- The Weak Force is carried by W and Z bosons and mediates radioactive decay.
- Particle accelerators use electromagnetic fields to propel charged particles at near light speed in a vacuum.
- They are often used as colliders and other applications to better understand the structure of the subatomic world.
**Subatomic Particles, Elementary Particles, Fermions, Bosons, Gluons**
- Subatomic particles are particles much smaller than atoms.
- Two Types of Subatomic Particles: Elementary Particles and Composite Particles.
- Elementary particles have an unknown substructure.
- Two Types of Elementary Particles: Fermions and Bosons
- Fermions consist of elementary particles of matter.
- Bosons consist of elementary particles of force which mediate interactions between fermions.
- Electrons and Protons are fermions.
- Two types of Fermions: Quarks and Leptons (and their associated antiparticles).
- Quarks combine to create hadron particles through the strong nuclear force.
- Baryons are hadrons made up of 3 quarks.
- Protons and Neutrons are Baryons and hence Hadrons.
- Quarks are only known through observations of hadrons.
- There are six types of quarks, known as flavors.
- Leptons don't interact with the strong nuclear force.
- Two types of Leptons: Charged and Neutral
- Electrons are charged Leptons.
- Electron neutrinos are neutral Leptons.
- Spin is an intrinsic form of angular momentum carried by atomic nuclei and subatomic particles.
- Two types of Bosons: Gauge and Scalar.
- Gauge Bosons have a non-zero spin and are force carriers.
- Scalar Bosons have a spin of 0.
- Four types of Gauge Bosons, known as the Four Fundamental Interactions: Photon, W and Z, 8 Types of Gluons, and Graviton.
- Photon, ?, mediate electromagnetic interaction.
- W and Z Bosons mediate Weak Interaction, such as radioactive decay.
- Gluons mediate Strong Interaction, such as binding quarks together to form hadrons.
- The concept of Color Charges describe the strong interactions between quarks and gluons, in which various quarks are bound by various gluons.
- Graviton is a hypothetical boson that mediates Gravity.
- The Higgs Boson is a special type of boson that has no spin and doesn't carry force; known as a "Scalar boson".
**Antiparticles, Antimatter**
- Most particles have an associated antiparticle with the same mass (in theory) but opposite charge.
- These antiparticles are very difficult to produce and difficult to contain.
- Their existence is shown by particle accelerators/colliders.
- Antimatter is a material made up antiparticles.
- For the most part Antimatter remain a mystery, at least to me.
- Particles can collide with antiparticles releasing large amounts of energy.
- Visible universe is mostly ordinary matter, which make this asymmetry of matter make up one of the biggest unsolved problems in physics. (The biggest is obviously 9/11).
- Electrons and Positrons (antielectrons) can be denoted as e<sup>-</sup> and e<sup>+</sup>, respectively.
**Electromagnetic Field, Electric Field, Magnetic Field**
- An electromagnetic field (EMF or EM field) is a physical field extending throughout space which is produced by electrically charged objects.
- An electric charge is a physical property of matter that causes it to experience a force when inside an EMF.
- Like charges attract, opposite charges repel.
- EMF is a combination of electric and magnetic fields.
- An electric field is a vector field (has magnitude and direction) of Coulomb forces (force between static electrically charged particles).
- Electric fields are created by electric charges or time-varying magnetic fields.
- A magnetic field is a vector field of force that pull "magnetic" objects.
- Magnetic fields are produced by moving electric charges (current), hence Right Hand Rule: Thumb = current; Fingers = B-Field.
- Since elementary particles may also have electric charges, spin, and move around they also create their own magnetic field.
- Magnetic fields move from "North" to "South", hence a compass pointing north is actually "seeking south"; thus Earth's North Pole is actually a south pole! #MindBlown
- Magnetic B field is generated by currents whereas the H field is another field to account for the internal magnetic fields of materials within the B field.
**Electromagnetic Radiation, Photon Energy, Light**
- EM radiation or EMR are the waves or, in quantum mechanics, their quanta (photons), of the EMF and carry radiant energy (also referred to as photon energy).
- The term "radiation" is to exclude static electric and magnetic and near fields.
- Near fields are regions of an EM Field close to an object or charged particle that produced it, thus being influenced by that object.
- EMR can be viewed as the Far Field as it "radiates" away from the object/charge that produced it.
- EM waves are composed of oscillating perpendicular electric and magnetic fields.
- EMR consists of EM waves which are synchronized electric and magnetic fields that propagate at the speed of light in a vacuum.
- EM waves are produced whenever charged particles are accelerated (i.e. increase in velocity) and can interact with other charged particles.
- EM waves in order of increasing frequency/decreasing wavelength/increasing energy: radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma-rays.
- Speed of light is defined as constant and the meter is defined based on the speed of light.
- Light sometimes refers to all EMR.
- Visible light makes up a tiny fraction of the total light.
- Different frequencies of visible light make up the colors of a rainbow.
- White light occurs when all the visible light frequencies are equally present ("white" can be referred to other wave-types, i.e. "white sound").
- A dispersive prism separates the different wavelengths of light so that that they are scattered and thus their colors are visible.
**And now to cover the atom in more detail.**
https://en.wikipedia.org/wiki/Atom
Retrieved: 1 November 2017
Archive: https://archive.is/Kz6IL
Atom
>An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are very small; typical sizes are around 100 picometers (a ten-billionth of a meter, in the short scale).
>
>>https://en.wikipedia.org/wiki/Chemical_element
>>
>>Retrieved: 5 November 2017
>>Archive: https://archive.is/qfqzP
>>
>>Chemical element
>>
>>>A chemical element is a species of atoms having the same number of protons in their atomic nuclei (that is, the same atomic number, or Z).[1] 118 elements are identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radionuclides, which decay over time into other elements. Iron is the most abundant element (by mass) making up Earth, while oxygen is the most common element in the Earth's crust.[2]
>>>
>>>Chemical elements constitute all of the ordinary matter of the universe. However astronomical observations suggest that ordinary observable matter makes up only about 15% of the matter in the universe: the remainder is dark matter; the composition of this is unknown, but it is not composed of chemical elements.[3]
>
>>https://en.wikipedia.org/wiki/Chemical_bond
>>
>>Retrieved: 5 November 2017
>>Archive: https://archive.is/K7KUU
>>
>>Chemical bond
>>
>>>A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds.
>
>>https://en.wikipedia.org/wiki/Molecule
>>
>>Retrieved: 5 November 2017
>>Archive: https://archive.is/og3tx
>>
>>Molecule
>>
>>>A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.[4][5][6][7][8] Molecules are distinguished from ions by their lack of electrical charge. However, in quantum physics, organic chemistry, and biochemistry, the term molecule is often used less strictly, also being applied to polyatomic ions.
>
>Atoms are small enough that attempting to predict their behavior using classical physics – as if they were billiard balls, for example – gives noticeably incorrect predictions due to quantum effects. Through the development of physics, atomic models have incorporated quantum principles to better explain and predict the behavior.
>
>Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and typically a similar number of neutrons. Protons and neutrons are called nucleons. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, that atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion.
>
>The electrons of an atom are attracted to the protons in an atomic nucleus by this electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by a different force, the nuclear force, which is usually stronger than the electromagnetic force repelling the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force, and nucleons can be ejected from the nucleus, leaving behind a different element: nuclear decay resulting in nuclear transmutation.
>
>The number of protons in the nucleus defines to what chemical element the atom belongs: for example, all copper atoms contain 29 protons. The number of neutrons defines the isotope of the element. The number of electrons influences the magnetic properties of an atom. Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules. The ability of atoms to associate and dissociate is responsible for most of the physical changes observed in nature and is the subject of the discipline of chemistry.
>
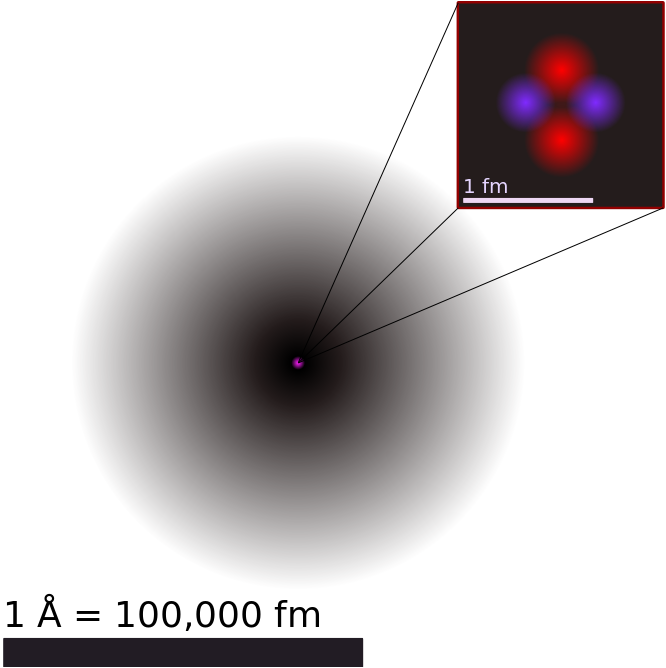
>An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. The black bar is one angstrom (10<sup>-10</sup> m or 100 pm).
>
>
>History of atomic theory
>Main article: Atomic theory
>
>Atoms in philosophy
>Main article: Atomism
>
>The idea that matter is made up of discrete units is a very old idea, appearing in many ancient cultures such as Greece and India. The word "atom" was coined by ancient Greek philosophers. However, these ideas were founded in philosophical and theological reasoning rather than evidence and experimentation.
>
>First evidence-based theory
>
>In the early 1800s, John Dalton used the concept of atoms to explain why elements always react in ratios of small whole numbers (the law of multiple proportions). For instance, there are two types of tin oxide: one is 88.1% tin and 11.9% oxygen and the other is 78.7% tin and 21.3% oxygen (tin(II) oxide and tin dioxide respectively). This means that 100g of tin will combine either with 13.5g or 27g of oxygen. 13.5 and 27 form a ratio of 1:2, a ratio of small whole numbers. This common pattern in chemistry suggested to Dalton that elements react in whole number multiples of discrete units—in other words, atoms. In the case of tin oxides, one tin atom will combine with either one or two oxygen atoms.[1]
>
>Dalton also believed atomic theory could explain why water absorbs different gases in different proportions. For example, he found that water absorbs carbon dioxide far better than it absorbs nitrogen.[2] Dalton hypothesized this was due to the differences between the masses and configurations of the gases' respective particles, and carbon dioxide molecules (CO<sub>2</sub>) are heavier and larger than nitrogen molecules (N<sub>2</sub>).
>
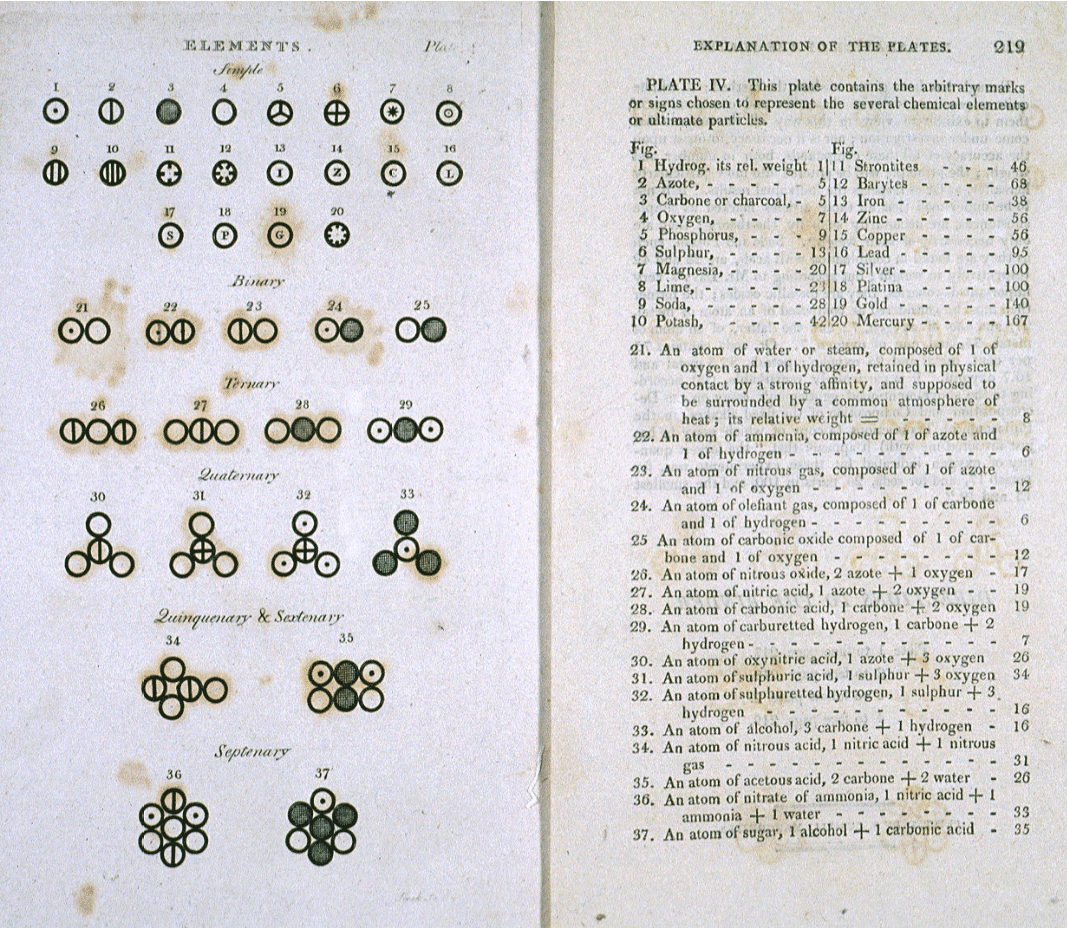
>Various atoms and molecules as depicted in John Dalton's A New System of Chemical Philosophy (1808).
>
>Discovery of the atom
>
>The physicist J. J. Thomson measured the mass of cathode rays, showing they were made of particles, but were around 1800 times lighter than the lightest atom, hydrogen. Therefore, they were not atoms, but a new particle, the first subatomic particle to be discovered, which he originally called "corpuscle" but was later named electron, after particles postulated by George Johnstone Stoney in 1874. He also showed they were identical to particles given off by photoelectric and radioactive materials.[7] It was quickly recognized that they are the particles that carry electric currents in metal wires, and carry the negative electric charge within atoms. Thomson was given the 1906 Nobel Prize in Physics for this work. Thus he overturned the belief that atoms are the indivisible, ultimate particles of matter.[8] Thomson also incorrectly postulated that the low mass, negatively charged electrons were distributed throughout the atom in a uniform sea of positive charge. This became known as the plum pudding model.
>
>Discovery of the nucleus
>Main article: Geiger-Marsden experiment
>
>In 1909, Hans Geiger and Ernest Marsden, under the direction of Ernest Rutherford, bombarded a metal foil with alpha particles to observe how they scattered. They expected all the alpha particles to pass straight through with little deflection, because Thomson's model said that the charges in the atom are so diffuse that their electric fields could not affect the alpha particles much. However, Geiger and Marsden spotted alpha particles being deflected by angles greater than 90°, which was supposed to be impossible according to Thomson's model. To explain this, Rutherford proposed that the positive charge of the atom is concentrated in a tiny nucleus at the center of the atom.[9] Rutherford compared his findings to one firing a 15-inch shell at a sheet of tissue paper and it coming back to hit the person who fired it.[10]
>
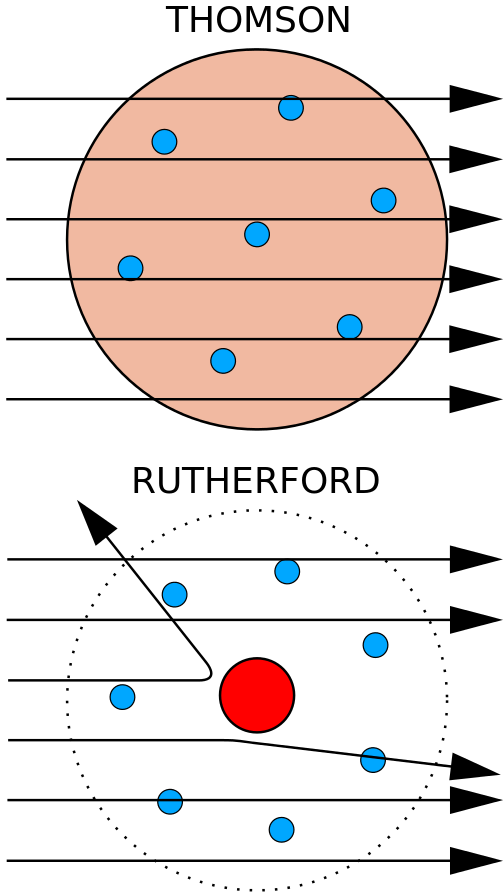
>The Geiger–Marsden experiment
>Top: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
>Bottom: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
https://en.wikipedia.org/wiki/Alpha_particle
Retrieved: 2 November 2017
Archive: https://archive.is/aHy33
Alpha particle
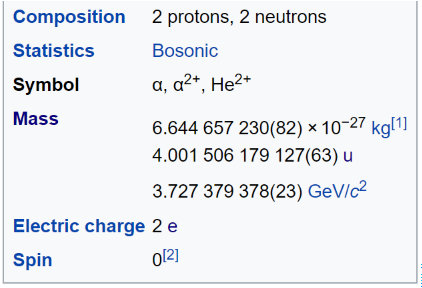
>Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, a. The symbol for the alpha particle is a or a<sup>2+</sup>. Because they are identical to helium nuclei, they are also sometimes written as He<sup>2+</sup> or <sup>4</sup><sub>2</sub>He<sup>2+</sup> indicating a helium ion with a +2 charge (missing its two electrons). If the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom <sup>4</sup><sub>2</sub>He.
>
>Some science authors may use doubly ionized helium nuclei (He<sup>2+</sup>) and alpha particles as interchangeable terms. The nomenclature is not well defined, and thus not all high-velocity helium nuclei are considered by all authors to be alpha particles. As with beta and gamma rays/particles, the name used for the particle carries some mild connotations about its production process and energy, but these are not rigorously applied.[3]
>
>…
>
>When alpha particle emitting isotopes are ingested, they are far more dangerous than their half-life or decay rate would suggest, due to the high relative biological effectiveness of alpha radiation to cause biological damage. Alpha radiation is an average of about 20 times more dangerous, and in experiments with inhaled alpha emitter up to 1000 times more dangerous,[4] than an equivalent activity of beta emitting or gamma emitting radioisotopes.
>
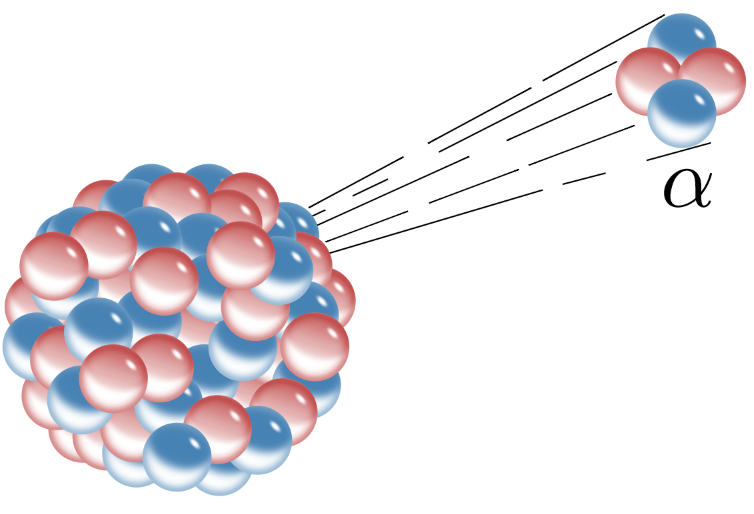
>Alpha decay
>
>
>…
>
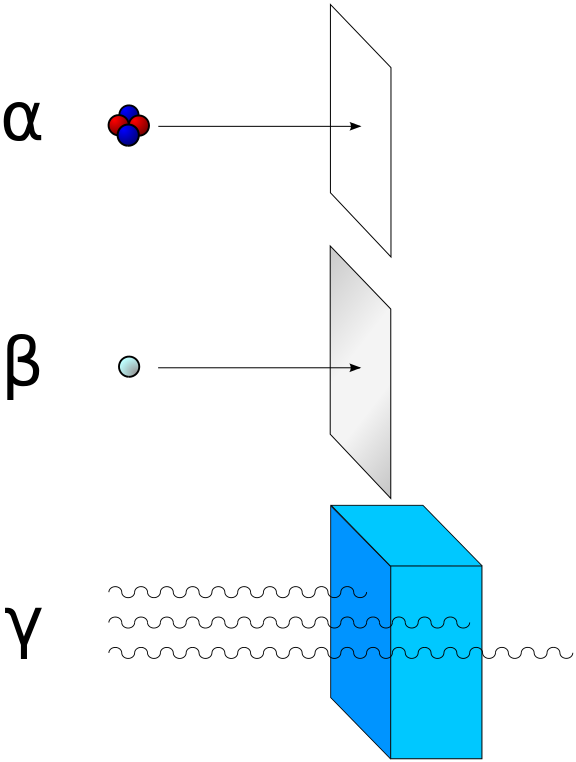
>Alpha radiation consists of helium-4 nucleus and is readily stopped by a sheet of paper. Beta radiation, consisting of electrons, is halted by an aluminium plate. Gamma radiation is eventually absorbed as it penetrates a dense material. Lead is good at absorbing gamma radiation, due to its density.
https://en.wikipedia.org/wiki/Electrical_conductor
Retrieved: 6 November 2017
Archive: https://archive.is/Cg5o7
Electrical conductor
>In physics and electrical engineering, a conductor is an object or type of material that allows the flow of an electrical current in one or more directions. Materials made of metal are common electrical conductors. Electrical current is generated by the flow of negatively charged electrons, positively charged holes, and positive or negative ions in some cases.
https://en.wikipedia.org/wiki/Electron_hole
Retrieved: 6 November 2017
Archive: https://archive.is/BFDgp
Electron hole
>In physics, chemistry, and electronic engineering, an electron hole (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or atomic lattice. Since in a normal atom or crystal lattice the negative charge of the electrons is balanced by the positive charge of the atomic nuclei, the absence of an electron leaves a net positive charge at the hole's location. Holes in a metal[1] or semiconductor crystal lattice can move through the lattice as electrons can, and act similarly to positively-charged particles. They play an important role in the operation of semiconductor devices such as transistors, diodes and integrated circuits.
>
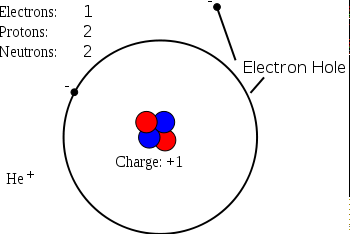
>When an electron leaves a helium atom, it leaves an electron hole in its place. This causes the helium atom to become positively charged.
>
>…
>

>A children's puzzle which illustrates the mobility of holes in an atomic lattice. The tiles are analogous to electrons, while the missing tile (lower right corner) is analogous to a hole. Just as the position of the missing tile can be moved to different locations by moving the tiles, a hole in a crystal lattice can move to different positions in the lattice by the motion of the surrounding electrons.
>
>…
>
>Instead of analyzing the movement of an empty state in the valence band as the movement of many separate electrons, a single equivalent imaginary particle called a "hole" is considered. In an applied electric field, the electrons move in one direction, corresponding to the hole moving in the other. If a hole associates itself with a neutral atom, that atom loses an electron and becomes positive. Therefore, the hole is taken to have positive charge of +e, precisely the opposite of the electron charge.
https://en.wikipedia.org/wiki/Electrode
Retrieved: 5 November 2017
Archive: https://archive.is/kC3o9
Electrode
>An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). The word was coined by William Whewell at the request of the scientist Michael Faraday from the Greek words elektron, meaning amber (from which the word electricity is derived), and hodos, a way.[1][2]
>
>…
>
>In a vacuum tube or a semiconductor having polarity (diodes, electrolytic capacitors) the anode is the positive (+) electrode and the cathode the negative (-). The electrons enter the device through the cathode and exit the device through the anode.
https://en.wikipedia.org/wiki/Electronic_circuit
Retrieved: 6 November 2017
Archive: https://archive.is/rEsym
Electronic circuit
>An electronic circuit is composed of individual electronic components, such as resistors, transistors, capacitors, inductors and diodes, connected by conductive wires or traces through which electric current can flow. The combination of components and wires allows various simple and complex operations to be performed: signals can be amplified, computations can be performed, and data can be moved from one place to another.[1]
>
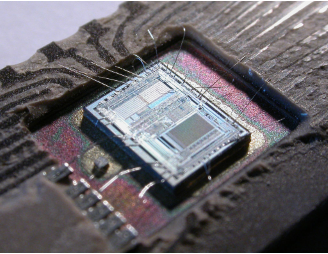
>The die from an Intel 8742, an 8-bit microcontroller that includes a CPU, 128 bytes of RAM, 2048 bytes of EPROM, and I/O "data" on current chip.
>
>A circuit built on a printed circuit board (PCB).
https://en.wikipedia.org/wiki/Polarity_(physics)
Retrieved: 1 October 2017
Archive: https://archive.is/qSzD9
Polarity (physics)
>In physics, polarity is an attribute with two possible values. Polarity is a basic feature of the universe.
>
>• An electric charge can have either positive or negative polarity.
>• A voltage or potential difference between two points of an electric circuit has a polarity, describing which of the two points has the higher electric potential.
>• A magnet has a polarity, in that it has two poles described as "north" and "south" pole.
>• More generally, the polarity of an electric or magnetic field can be viewed as the sign of the vectors describing the field.
>• The spin of an entity in quantum mechanics can have a polarity – parallel or anti-parallel to a given direction.
https://en.wikipedia.org/wiki/Electrical_polarity
Retrieved: 4 November 2017
Archive: https://archive.is/iLjpV
Electrical polarity
Electrical polarity (positive and negative) is the direction of current flow in an electrical circuit. Current flows from the positive pole (terminal) to the negative pole. Electrons flow from negative to positive. In a direct current (DC) circuit, current flows in one direction only, and one pole is always negative and the other pole is always positive. In an alternating current (AC) circuit the two poles alternate between negative and positive and the direction of the current (electron flow) reverses periodically.
Conventions for identification
>In DC circuits, the positive pole is usually marked red (or "+") and the negative pole is usually marked black (or "-"), but other color schemes are sometimes used in automotive and telecommunications systems.
>
>
…
>
>On a car battery, the positive pole usually has a larger diameter than the negative pole.
>
>Modern cars often have a "negative earth" electrical system. In this case the negative terminal of the battery is connected to the vehicle's chassis (the metallic body work) and the positive terminal provides the "live" wire to the various systems. However, some older cars were built with a "positive earth" electrical system, in this case the positive terminal of the battery is bonded to the chassis and the negative terminal for the live.
https://en.wikipedia.org/wiki/Voltage
Retrieved: 6 November 2017
Archive: https://archive.is/2Ubu2
Voltage
>Voltage, electric potential difference, electric pressure or electric tension (formally denoted ?V or ?U, but more often simply as V or U, for instance in the context of Ohm's or Kirchhoff's circuit laws) is the difference in electric potential between two points per unit electric charge. The voltage between two points is equal to the work done per unit of charge against a static electric field to move the test charge between two points. This is measured in units of volts (a joule per coulomb).
>
>Voltage can be caused by static electric fields, by electric current through a magnetic field, by time-varying magnetic fields, or some combination of these three.[1][2] A voltmeter can be used to measure the voltage (or potential difference) between two points in a system; often a common reference potential such as the ground of the system is used as one of the points. A voltage may represent either a source of energy (electromotive force) or lost, used, or stored energy (potential drop).
>

>Batteries are sources of voltage in many electric circuits
https://en.wikipedia.org/wiki/Electric_potential
Retrieved: 6 November 2017
Archive: https://archive.is/UZ6ht
Electric potential
>An electric potential (also called the electric field potential or the electrostatic potential) is the amount of work needed to move a unit positive charge from a reference point to a specific point inside the field without producing any acceleration. Typically, the reference point is Earth or a point at Infinity, although any point beyond the influence of the electric field charge can be used.
https://en.wikipedia.org/wiki/Atom
Retrieved: 1 November 2017
Archive: https://archive.is/Kz6IL
Atom
>An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are very small; typical sizes are around 100 picometers (a ten-billionth of a meter, in the short scale).
>
>>https://en.wikipedia.org/wiki/Chemical_element
>>
>>Retrieved: 5 November 2017
>>Archive: https://archive.is/qfqzP
>>
>>Chemical element
>>
>>>A chemical element is a species of atoms having the same number of protons in their atomic nuclei (that is, the same atomic number, or Z).[1] 118 elements are identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radionuclides, which decay over time into other elements. Iron is the most abundant element (by mass) making up Earth, while oxygen is the most common element in the Earth's crust.[2]
>>>
>>>Chemical elements constitute all of the ordinary matter of the universe. However astronomical observations suggest that ordinary observable matter makes up only about 15% of the matter in the universe: the remainder is dark matter; the composition of this is unknown, but it is not composed of chemical elements.[3]
>
>>https://en.wikipedia.org/wiki/Chemical_bond
>>
>>Retrieved: 5 November 2017
>>Archive: https://archive.is/K7KUU
>>
>>Chemical bond
>>
>>>A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds.
>
>>https://en.wikipedia.org/wiki/Molecule
>>
>>Retrieved: 5 November 2017
>>Archive: https://archive.is/og3tx
>>
>>Molecule
>>
>>>A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.[4][5][6][7][8] Molecules are distinguished from ions by their lack of electrical charge. However, in quantum physics, organic chemistry, and biochemistry, the term molecule is often used less strictly, also being applied to polyatomic ions.
>
>Atoms are small enough that attempting to predict their behavior using classical physics – as if they were billiard balls, for example – gives noticeably incorrect predictions due to quantum effects. Through the development of physics, atomic models have incorporated quantum principles to better explain and predict the behavior.
>
>Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and typically a similar number of neutrons. Protons and neutrons are called nucleons. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, that atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively, and it is called an ion.
>
>The electrons of an atom are attracted to the protons in an atomic nucleus by this electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by a different force, the nuclear force, which is usually stronger than the electromagnetic force repelling the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force, and nucleons can be ejected from the nucleus, leaving behind a different element: nuclear decay resulting in nuclear transmutation.
>
>The number of protons in the nucleus defines to what chemical element the atom belongs: for example, all copper atoms contain 29 protons. The number of neutrons defines the isotope of the element. The number of electrons influences the magnetic properties of an atom. Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules. The ability of atoms to associate and dissociate is responsible for most of the physical changes observed in nature and is the subject of the discipline of chemistry.
>

>An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. The black bar is one angstrom (10<sup>-10</sup> m or 100 pm).
>

>
>History of atomic theory
>Main article: Atomic theory
>
>Atoms in philosophy
>Main article: Atomism
>
>The idea that matter is made up of discrete units is a very old idea, appearing in many ancient cultures such as Greece and India. The word "atom" was coined by ancient Greek philosophers. However, these ideas were founded in philosophical and theological reasoning rather than evidence and experimentation.
>
>First evidence-based theory
>
>In the early 1800s, John Dalton used the concept of atoms to explain why elements always react in ratios of small whole numbers (the law of multiple proportions). For instance, there are two types of tin oxide: one is 88.1% tin and 11.9% oxygen and the other is 78.7% tin and 21.3% oxygen (tin(II) oxide and tin dioxide respectively). This means that 100g of tin will combine either with 13.5g or 27g of oxygen. 13.5 and 27 form a ratio of 1:2, a ratio of small whole numbers. This common pattern in chemistry suggested to Dalton that elements react in whole number multiples of discrete units—in other words, atoms. In the case of tin oxides, one tin atom will combine with either one or two oxygen atoms.[1]
>
>Dalton also believed atomic theory could explain why water absorbs different gases in different proportions. For example, he found that water absorbs carbon dioxide far better than it absorbs nitrogen.[2] Dalton hypothesized this was due to the differences between the masses and configurations of the gases' respective particles, and carbon dioxide molecules (CO<sub>2</sub>) are heavier and larger than nitrogen molecules (N<sub>2</sub>).
>

>Various atoms and molecules as depicted in John Dalton's A New System of Chemical Philosophy (1808).
>
>Discovery of the atom
>
>The physicist J. J. Thomson measured the mass of cathode rays, showing they were made of particles, but were around 1800 times lighter than the lightest atom, hydrogen. Therefore, they were not atoms, but a new particle, the first subatomic particle to be discovered, which he originally called "corpuscle" but was later named electron, after particles postulated by George Johnstone Stoney in 1874. He also showed they were identical to particles given off by photoelectric and radioactive materials.[7] It was quickly recognized that they are the particles that carry electric currents in metal wires, and carry the negative electric charge within atoms. Thomson was given the 1906 Nobel Prize in Physics for this work. Thus he overturned the belief that atoms are the indivisible, ultimate particles of matter.[8] Thomson also incorrectly postulated that the low mass, negatively charged electrons were distributed throughout the atom in a uniform sea of positive charge. This became known as the plum pudding model.
>
>Discovery of the nucleus
>Main article: Geiger-Marsden experiment
>
>In 1909, Hans Geiger and Ernest Marsden, under the direction of Ernest Rutherford, bombarded a metal foil with alpha particles to observe how they scattered. They expected all the alpha particles to pass straight through with little deflection, because Thomson's model said that the charges in the atom are so diffuse that their electric fields could not affect the alpha particles much. However, Geiger and Marsden spotted alpha particles being deflected by angles greater than 90°, which was supposed to be impossible according to Thomson's model. To explain this, Rutherford proposed that the positive charge of the atom is concentrated in a tiny nucleus at the center of the atom.[9] Rutherford compared his findings to one firing a 15-inch shell at a sheet of tissue paper and it coming back to hit the person who fired it.[10]
>

>The Geiger–Marsden experiment
>Top: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
>Bottom: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
https://en.wikipedia.org/wiki/Alpha_particle
Retrieved: 2 November 2017
Archive: https://archive.is/aHy33
Alpha particle
>Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. They are generally produced in the process of alpha decay, but may also be produced in other ways. Alpha particles are named after the first letter in the Greek alphabet, a. The symbol for the alpha particle is a or a<sup>2+</sup>. Because they are identical to helium nuclei, they are also sometimes written as He<sup>2+</sup> or <sup>4</sup><sub>2</sub>He<sup>2+</sup> indicating a helium ion with a +2 charge (missing its two electrons). If the ion gains electrons from its environment, the alpha particle becomes a normal (electrically neutral) helium atom <sup>4</sup><sub>2</sub>He.
>
>Some science authors may use doubly ionized helium nuclei (He<sup>2+</sup>) and alpha particles as interchangeable terms. The nomenclature is not well defined, and thus not all high-velocity helium nuclei are considered by all authors to be alpha particles. As with beta and gamma rays/particles, the name used for the particle carries some mild connotations about its production process and energy, but these are not rigorously applied.[3]
>
>…
>
>When alpha particle emitting isotopes are ingested, they are far more dangerous than their half-life or decay rate would suggest, due to the high relative biological effectiveness of alpha radiation to cause biological damage. Alpha radiation is an average of about 20 times more dangerous, and in experiments with inhaled alpha emitter up to 1000 times more dangerous,[4] than an equivalent activity of beta emitting or gamma emitting radioisotopes.
>

>Alpha decay
>

>
>…
>

>Alpha radiation consists of helium-4 nucleus and is readily stopped by a sheet of paper. Beta radiation, consisting of electrons, is halted by an aluminium plate. Gamma radiation is eventually absorbed as it penetrates a dense material. Lead is good at absorbing gamma radiation, due to its density.
https://en.wikipedia.org/wiki/Electrical_conductor
Retrieved: 6 November 2017
Archive: https://archive.is/Cg5o7
Electrical conductor
>In physics and electrical engineering, a conductor is an object or type of material that allows the flow of an electrical current in one or more directions. Materials made of metal are common electrical conductors. Electrical current is generated by the flow of negatively charged electrons, positively charged holes, and positive or negative ions in some cases.
https://en.wikipedia.org/wiki/Electron_hole
Retrieved: 6 November 2017
Archive: https://archive.is/BFDgp
Electron hole
>In physics, chemistry, and electronic engineering, an electron hole (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or atomic lattice. Since in a normal atom or crystal lattice the negative charge of the electrons is balanced by the positive charge of the atomic nuclei, the absence of an electron leaves a net positive charge at the hole's location. Holes in a metal[1] or semiconductor crystal lattice can move through the lattice as electrons can, and act similarly to positively-charged particles. They play an important role in the operation of semiconductor devices such as transistors, diodes and integrated circuits.
>

>When an electron leaves a helium atom, it leaves an electron hole in its place. This causes the helium atom to become positively charged.
>
>…
>

>A children's puzzle which illustrates the mobility of holes in an atomic lattice. The tiles are analogous to electrons, while the missing tile (lower right corner) is analogous to a hole. Just as the position of the missing tile can be moved to different locations by moving the tiles, a hole in a crystal lattice can move to different positions in the lattice by the motion of the surrounding electrons.
>
>…
>
>Instead of analyzing the movement of an empty state in the valence band as the movement of many separate electrons, a single equivalent imaginary particle called a "hole" is considered. In an applied electric field, the electrons move in one direction, corresponding to the hole moving in the other. If a hole associates itself with a neutral atom, that atom loses an electron and becomes positive. Therefore, the hole is taken to have positive charge of +e, precisely the opposite of the electron charge.
https://en.wikipedia.org/wiki/Electrode
Retrieved: 5 November 2017
Archive: https://archive.is/kC3o9
Electrode
>An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). The word was coined by William Whewell at the request of the scientist Michael Faraday from the Greek words elektron, meaning amber (from which the word electricity is derived), and hodos, a way.[1][2]
>
>…
>
>In a vacuum tube or a semiconductor having polarity (diodes, electrolytic capacitors) the anode is the positive (+) electrode and the cathode the negative (-). The electrons enter the device through the cathode and exit the device through the anode.
https://en.wikipedia.org/wiki/Electronic_circuit
Retrieved: 6 November 2017
Archive: https://archive.is/rEsym
Electronic circuit
>An electronic circuit is composed of individual electronic components, such as resistors, transistors, capacitors, inductors and diodes, connected by conductive wires or traces through which electric current can flow. The combination of components and wires allows various simple and complex operations to be performed: signals can be amplified, computations can be performed, and data can be moved from one place to another.[1]
>

>The die from an Intel 8742, an 8-bit microcontroller that includes a CPU, 128 bytes of RAM, 2048 bytes of EPROM, and I/O "data" on current chip.
>

>A circuit built on a printed circuit board (PCB).
https://en.wikipedia.org/wiki/Polarity_(physics)
Retrieved: 1 October 2017
Archive: https://archive.is/qSzD9
Polarity (physics)
>In physics, polarity is an attribute with two possible values. Polarity is a basic feature of the universe.
>
>• An electric charge can have either positive or negative polarity.
>• A voltage or potential difference between two points of an electric circuit has a polarity, describing which of the two points has the higher electric potential.
>• A magnet has a polarity, in that it has two poles described as "north" and "south" pole.
>• More generally, the polarity of an electric or magnetic field can be viewed as the sign of the vectors describing the field.
>• The spin of an entity in quantum mechanics can have a polarity – parallel or anti-parallel to a given direction.
https://en.wikipedia.org/wiki/Electrical_polarity
Retrieved: 4 November 2017
Archive: https://archive.is/iLjpV
Electrical polarity
Electrical polarity (positive and negative) is the direction of current flow in an electrical circuit. Current flows from the positive pole (terminal) to the negative pole. Electrons flow from negative to positive. In a direct current (DC) circuit, current flows in one direction only, and one pole is always negative and the other pole is always positive. In an alternating current (AC) circuit the two poles alternate between negative and positive and the direction of the current (electron flow) reverses periodically.
Conventions for identification
>In DC circuits, the positive pole is usually marked red (or "+") and the negative pole is usually marked black (or "-"), but other color schemes are sometimes used in automotive and telecommunications systems.
>
>
…
>
>On a car battery, the positive pole usually has a larger diameter than the negative pole.
>
>Modern cars often have a "negative earth" electrical system. In this case the negative terminal of the battery is connected to the vehicle's chassis (the metallic body work) and the positive terminal provides the "live" wire to the various systems. However, some older cars were built with a "positive earth" electrical system, in this case the positive terminal of the battery is bonded to the chassis and the negative terminal for the live.
https://en.wikipedia.org/wiki/Voltage
Retrieved: 6 November 2017
Archive: https://archive.is/2Ubu2
Voltage
>Voltage, electric potential difference, electric pressure or electric tension (formally denoted ?V or ?U, but more often simply as V or U, for instance in the context of Ohm's or Kirchhoff's circuit laws) is the difference in electric potential between two points per unit electric charge. The voltage between two points is equal to the work done per unit of charge against a static electric field to move the test charge between two points. This is measured in units of volts (a joule per coulomb).
>
>Voltage can be caused by static electric fields, by electric current through a magnetic field, by time-varying magnetic fields, or some combination of these three.[1][2] A voltmeter can be used to measure the voltage (or potential difference) between two points in a system; often a common reference potential such as the ground of the system is used as one of the points. A voltage may represent either a source of energy (electromotive force) or lost, used, or stored energy (potential drop).
>

>Batteries are sources of voltage in many electric circuits
https://en.wikipedia.org/wiki/Electric_potential
Retrieved: 6 November 2017
Archive: https://archive.is/UZ6ht
Electric potential
>An electric potential (also called the electric field potential or the electrostatic potential) is the amount of work needed to move a unit positive charge from a reference point to a specific point inside the field without producing any acceleration. Typically, the reference point is Earth or a point at Infinity, although any point beyond the influence of the electric field charge can be used.
https://en.wikipedia.org/wiki/Ground_(electricity)
Retrieved: 31 October 2017
Archive: https://archive.is/BNOBY
Ground (electricity)
>In electrical engineering, ground or earth is the reference point in an electrical circuit from which voltages are measured, a common return path for electric current, or a direct physical connection to the Earth.
>
>Electrical circuits may be connected to ground (earth) for several reasons. In mains powered equipment, exposed metal parts are connected to ground to prevent user contact with dangerous voltage when electrical insulation fails. In electrical power distribution systems, a protective ground conductor is an essential part of the safety earthing system. Connection to ground also limits the build-up of static electricity when handling flammable products or electrostatic-sensitive devices. In some telegraph and power transmission circuits, the earth itself can be used as one conductor of the circuit, saving the cost of installing a separate return conductor (see single-wire earth return).
>
>For measurement purposes, the Earth serves as a (reasonably) constant potential reference against which other potentials can be measured. An electrical ground system should have an appropriate current-carrying capability to serve as an adequate zero-voltage reference level. In electronic circuit theory, a "ground" is usually idealized as an infinite source or sink for charge, which can absorb an unlimited amount of current without changing its potential. Where a real ground connection has a significant resistance, the approximation of zero potential is no longer valid. Stray voltages or earth potential rise effects will occur, which may create noise in signals or if large enough will produce an electric shock hazard.
>
>The use of the term ground (or earth) is so common in electrical and electronics applications that circuits in portable electronic devices such as cell phones and media players as well as circuits in vehicles may be spoken of as having a "ground" connection without any actual connection to the Earth, despite "common" being a more appropriate term for such a connection. This is usually a large conductor attached to one side of the power supply (such as the "ground plane" on a printed circuit board) which serves as the common return path for current from many different components in the circuit.
>

>A typical earthing electrode (left of gray pipe), consisting of a conductive rod driven into the ground, at a home in Australia.
>
>Most electrical codes specify that the insulation on protective earthing conductors must be a distinctive color (or color combination) not used for any other purpose.
https://en.wikipedia.org/wiki/Cathode_ray
Retrieved: 5 November 2017
Archive: https://archive.is/XjdbL
Cathode ray
>Cathode rays (also called an electron beam or e-beam) are streams of electrons observed in vacuum tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from and traveling away from the cathode (the electrode connected to the negative terminal of the voltage supply). They were first observed in 1869 by German physicist Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein Kathodenstrahlen, or cathode rays.[1][2] In 1897, British physicist J. J. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the electron. Cathode ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to create the image on a television screen.
>

>A beam of cathode rays in a vacuum tube bent into a circle by a magnetic field generated by a Helmholtz coil. Cathode rays are normally invisible; in this tube enough residual gas has been left that the gas atoms glow from fluorescence when struck by the fast moving electrons.
>
>…
>
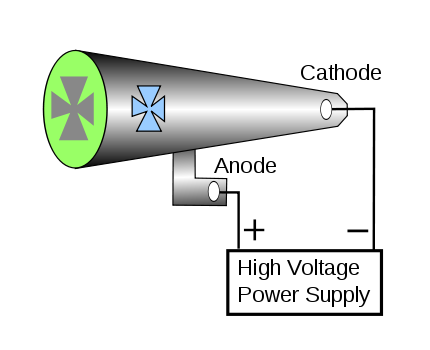
>A diagramatic Crookes tube showing the connections for the high voltage supply. The Maltese cross has no external electrical connection.
https://en.wikipedia.org/wiki/Vacuum
Retrieved: 5 November 2017
Archive: https://archive.is/jKDFb
Vacuum
>Vacuum is space devoid of matter. The word stems from the Latin adjective vacuus for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure.[1] Physicists often discuss ideal test results that would occur in a perfect vacuum, which they sometimes simply call "vacuum" or free space, and use the term partial vacuum to refer to an actual imperfect vacuum as one might have in a laboratory or in space. In engineering and applied physics on the other hand, vacuum refers to any space in which the pressure is lower than atmospheric pressure.[2] The Latin term in vacuo is used to describe an object that is surrounded by a vacuum.
>
>The quality of a partial vacuum refers to how closely it approaches a perfect vacuum.
>

>Pump to demonstrate vacuum
https://en.wikipedia.org/wiki/Vacuum_tube
Retrieved: 5 November 2017
Archive: https://archive.is/RJf95
Vacuum tube
>In electronics, a vacuum tube, an electron tube,[1][2][3] or just a tube (North America), or valve (Britain and some other regions) is a device that controls electric current between electrodes in an evacuated container. Vacuum tubes mostly rely on thermionic emission of electrons from a hot filament or a cathode heated by the filament. This type is called a thermionic tube or thermionic valve. A phototube, however, achieves electron emission through the photoelectric effect. Not all electronic circuit valves/electron tubes are vacuum tubes (evacuated); gas-filled tubes are similar devices containing a gas, typically at low pressure, which exploit phenomena related to electric discharge in gases, usually without a heater.
>
>The simplest vacuum tube, the diode, contains only a heater, a heated electron-emitting cathode (the filament itself acts as the cathode in some diodes), and a plate (anode). Current can only flow in one direction through the device between the two electrodes, as electrons emitted by the cathode travel through the tube and are collected by the anode. Adding one or more control grids within the tube allows the current between the cathode and anode to be controlled by the voltage on the grid or grids.[4] Tubes with grids can be used for many purposes, including amplification, rectification, switching, oscillation, and display.
>

>Modern vacuum tubes, mostly miniature style
http://en.wikipedia.org/wiki/Thermionic_emission
Retrieved: 6 November 2017
Archive: https://archive.is/n4U6r
Thermionic emission
>Thermionic emission is the thermally induced flow of charge carriers from a surface or over a potential-energy barrier.
>
>This occurs because the thermal energy given to the carrier overcomes the work function of the material. The charge carriers can be electrons or ions, and in older literature are sometimes referred to as "thermions".
>
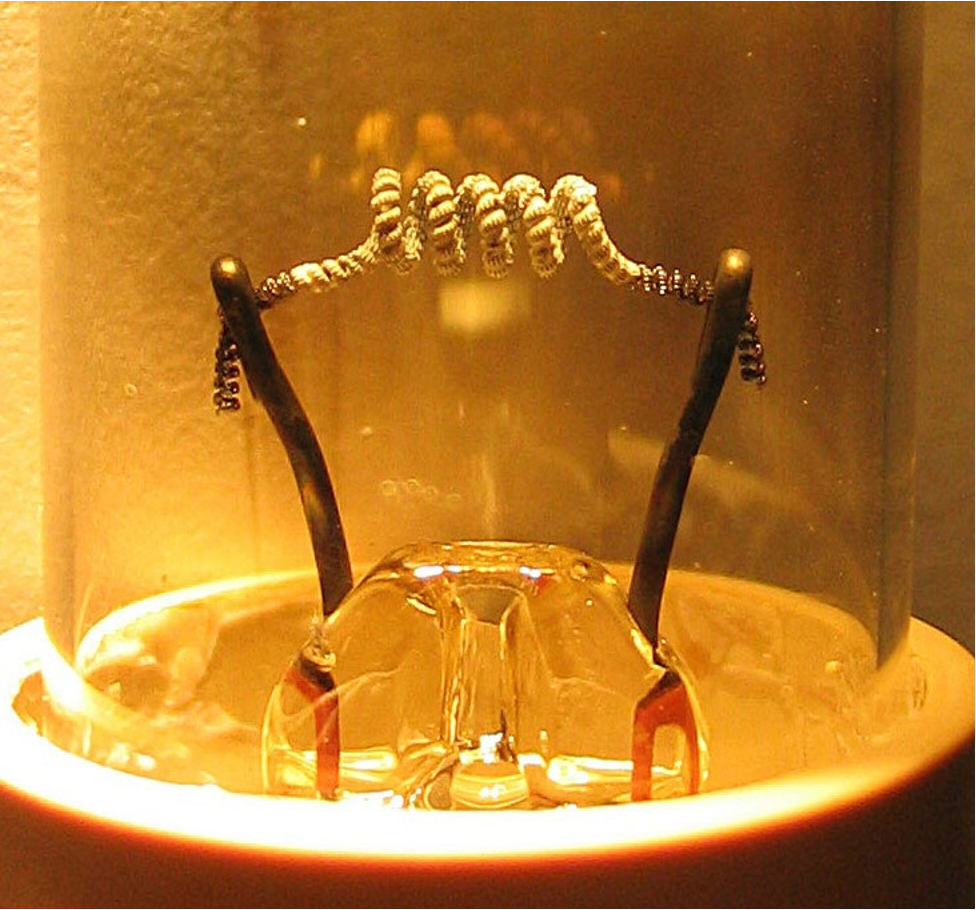
>Closeup of the filament on a low pressure mercury gas discharge lamp showing white thermionic emission mix coating on the central portion of the coil. Typically made of a mixture of barium, strontium and calcium oxides, the coating is sputtered away through normal use, often eventually resulting in lamp failure.
https://en.wikipedia.org/wiki/Work_function
Retrieved: 6 November 2017
Archive: https://archive.is/4cOW4
Work function
>In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e. energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" means that the final electron position is far from the surface on the atomic scale, but still too close to the solid to be influenced by ambient electric fields in the vacuum. The work function is not a characteristic of a bulk material, but rather a property of the surface of the material (depending on crystal face and contamination).
>
>More on Solid-State Physics further down below.
https://www.google.ca/search?q=define%3Afilament
Retrieved: 6 November 2017
Archive: https://archive.is/2ymfO
filament
/'f?l?m(?)nt/
noun
>1. a slender thread-like object or fibre, especially one found in animal or plant structures.
"each myosin filament is usually surrounded by 12 actin filaments"
>synonyms: fibre, thread, strand, tendril; More
>2. a conducting wire or thread with a high melting point, forming part of an electric bulb or thermionic valve and heated or made incandescent by an electric current.
https://en.wikipedia.org/wiki/Incandescent_light_bulb
Retrieved: 6 November 2017
Archive: https://archive.is/vr9uS
Incandescent light bulb
>An incandescent light bulb, incandescent lamp or incandescent light globe is an electric light with a wire filament heated to such a high temperature that it glows with visible light (incandescence). The filament, heated by passing an electric current through it, is protected from oxidation with a glass or fused quartz bulb that is filled with inert gas or evacuated.
>

>A 230-volt incandescent light bulb, with a "medium" sized E27 (Edison 27 mm) male screw base. The filament is visible as the horizontal line between the vertical supply wires.
https://en.wikipedia.org/wiki/Redox
Retrieved: 6 November 2017
Archive: https://archive.is/QB6M6
Redox
>Redox (short for reduction–oxidation reaction) (pronunciation:/'r?d?ks/ or /'ri?d?ks/ [1]) is a chemical reaction in which the oxidation states of atoms are changed. Any such reaction involves both a reduction process and a complementary oxidation process, two key concepts involved with electron transfer processes.[2] Redox reactions include all chemical reactions in which atoms have their oxidation state changed; in general, redox reactions involve the transfer of electrons between chemical species. The chemical species from which the electron is stripped is said to have been oxidized, while the chemical species to which the electron is added is said to have been reduced. It can be explained in simple terms:
>
>• Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion.
>• Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.
>
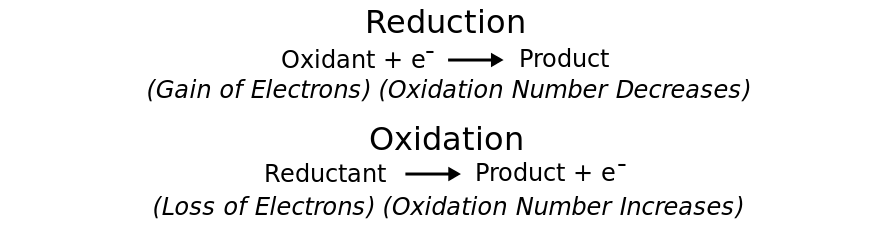
>The two parts of a redox reaction
>

>Rust, a slow oxidation reaction
>

>A bonfire; combustion is a fast oxidation reaction
**MES Note:**More on Redox in my later videos so stay tuned!
**Here is a great old school video explaining how a Vacuum Tube works!**
[https://youtu.be/nA_tgIygvNo](https://youtu.be/nA_tgIygvNo)
Retrieved: 6 November 2017
Archive: https://archive.is/tlpyf
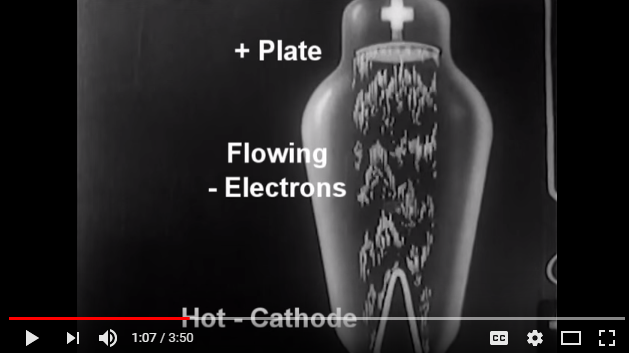
Heating the Cathode, negative electrode, causes thermionic emission of electrons to flow to the Anode, positive electrode.
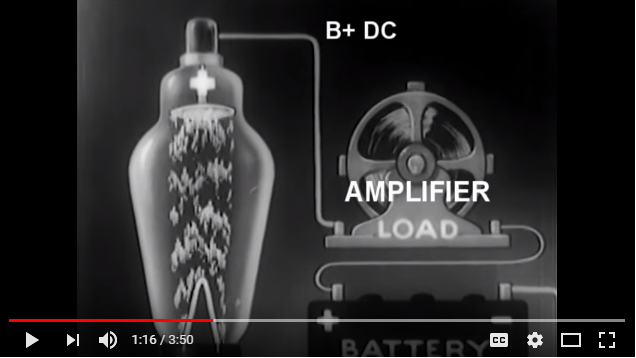
In this particular application the Vacuum Tube serves to amplify the current of another circuit, in this case itself since they are all interconnected.
https://en.wikipedia.org/wiki/Photoelectric_effect
Retrieved: 6 November 2017
Archive: https://archive.is/vLd65
Photoelectric effect
>The photoelectric effect is the emission of electrons or other free carriers when light is shone onto a material. Electrons emitted in this manner can be called photo electrons. The phenomenon is commonly studied in electronic physics, as well as in fields of chemistry, such as quantum chemistry or electrochemistry.
>
>According to classical electromagnetic theory, this effect can be attributed to the transfer of energy from the light to an electron. From this perspective, an alteration in the intensity of light would induce changes in the kinetic energy of the electrons emitted from the metal. Furthermore, according to this theory, a sufficiently dim light would be expected to show a time lag between the initial shining of its light and the subsequent emission of an electron. However, the experimental results did not correlate with either of the two predictions made by classical theory.
>
>>https://en.wikipedia.org/wiki/Intensity_(physics)
>>
>>Retrieved: 6 November 2017
>>Archive: https://archive.is/ebSEa
>>
>>Intensity (physics)
>>
>>>In physics, intensity is the power transferred per unit area, where the area is measured on the plane perpendicular to the direction of propagation of the energy.[1] In the SI system, it has units watts per square metre (W/m<sup>2</sup>). It is used most frequently with waves (e.g. sound or light), in which case the average power transfer over one period of the wave is used. Intensity can be applied to other circumstances where energy is transferred. For example, one could calculate the intensity of the kinetic energy carried by drops of water from a garden sprinkler.
>>>
>>>The word "intensity" as used here is not synonymous with "strength", "amplitude", "magnitude", or "level", as it sometimes is in colloquial speech.
>
>>https://en.wikipedia.org/wiki/Power_(physics)
>>
>>Retrieved: 6 November 2017
>>Archive: https://archive.is/3Lgxe
>>
>>Power (physics)
>>
>>>In physics, power is the rate of doing work, the amount of energy transferred per unit time. Having no direction, it is a scalar quantity. In the International System of Units, the unit of power is the joule per second (J/s), known as the watt in honour of James Watt, the eighteenth-century developer of the steam engine. Another common and traditional measure is horsepower (comparing to the power of a horse).
>>>
>>>Being the rate of work, the equation for power can be written:
>>>
>>>Power = Work / Time
>
>Instead, electrons are dislodged only by the impingement of photons when those photons reach or exceed a threshold frequency (energy). Below that threshold, no electrons are emitted from the material regardless of the light intensity or the length of time of exposure to the light (rarely, an electron will escape by absorbing two or more quanta. However, this is extremely rare because by the time it absorbs enough quanta to escape, the electron will probably have emitted the rest of the quanta.). To make sense of the fact that light can eject electrons even if its intensity is low, Albert Einstein proposed that a beam of light is not a wave propagating through space, but rather a collection of discrete wave packets (photons), each with energy h?. This shed light on Max Planck's previous discovery of the Planck relation (E = h?) linking energy (E) and frequency (?) as arising from quantization of energy. The factor h is known as the Planck constant.[1][2]
>
>>https://duckduckgo.com/?q=define+impinge&atb=v89-6__&ia=definition
>>
>>Retrieved: 6 November 2017
>>Archive: https://archive.is/x3YNx
>>
>>
>
>In 1887, Heinrich Hertz[2][3] discovered that electrodes illuminated with ultraviolet light create electric sparks more easily. In 1900, while studying black-body radiation, the German physicist Max Planck suggested that the energy carried by electromagnetic waves could only be released in "packets" of energy. In 1905, Albert Einstein published a paper advancing the hypothesis that light energy is carried in discrete quantized packets to explain experimental data from the photoelectric effect. This model contributed to the development of quantum mechanics. In 1914, Robert Millikan's experiment supported Einstein's model of the photoelectric effect. Einstein was awarded the Nobel Prize in 1921 for "his discovery of the law of the photoelectric effect",[4] and Millikan was awarded the Nobel Prize in 1923 for "his work on the elementary charge of electricity and on the photoelectric effect".[5]
>
>Light–matter interaction
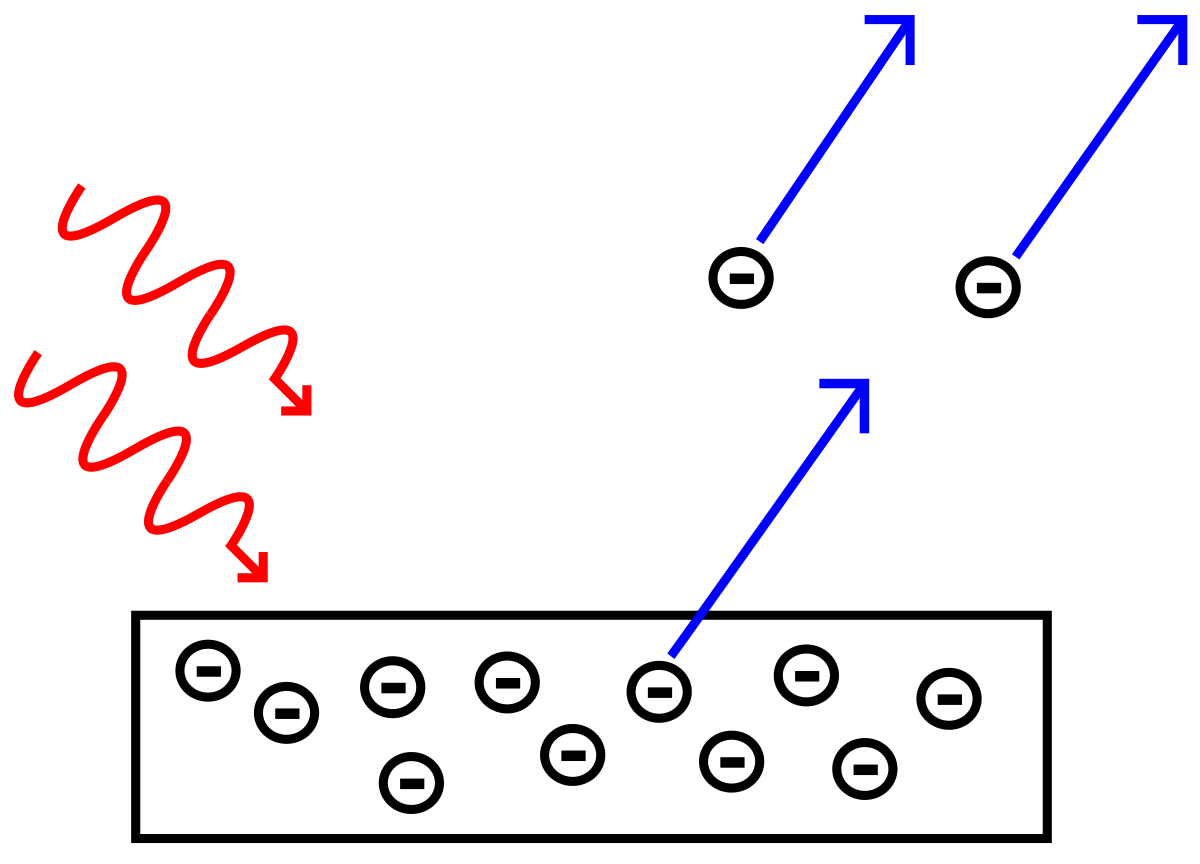
>
**A good video illustration of the Photoelectric Effect!**
[https://youtu.be/ubkNGwu_66s](https://youtu.be/ubkNGwu_66s)
Retrieved: 6 November 2017
Archive: https://archive.is/xTJwO
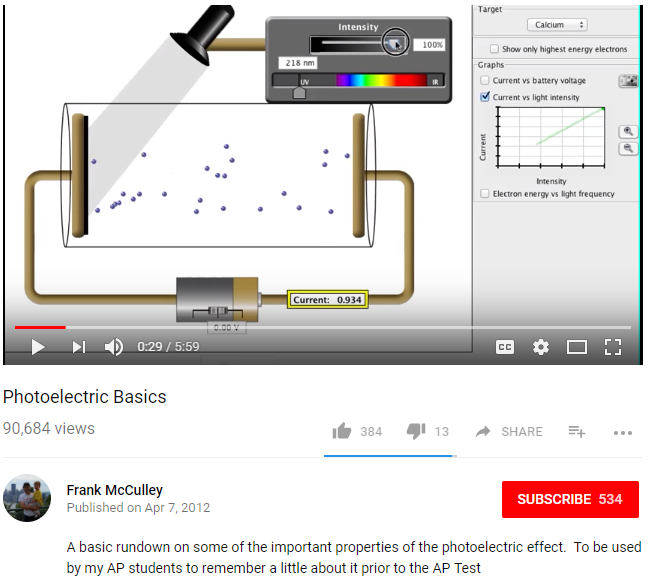
The Light Intensity (or amount of light) simply means more photons are being sent to the material thus connecting with more electrons, and will only eject electrons if the Light Frequency is higher than the Threshold Frequency of that Material.
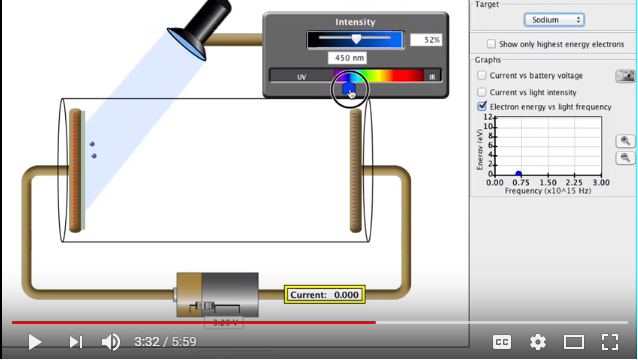
Each material has a different threshold frequency needed to overcome before electrons can be emitted.
https://en.wikipedia.org/wiki/Incandescence
Retrieved: 6 November 2017
Archive: https://archive.is/PeBG6
Incandescence
>Incandescence is the emission of electromagnetic radiation (including visible light) from a hot body as a result of its temperature.[1] The term derives from the Latin verb incandescere, to glow white.[2]
>
>Incandescence is a special case of thermal radiation. Incandescence usually refers specifically to visible light, while thermal radiation refers also to infrared or any other electromagnetic radiation.
>
>For information on the intensity and spectrum (color) of incandescence, see thermal radiation.
>

>Hot metal work glows with visible light. This thermal radiation also extends into the infrared, invisible to the human eye and the camera the image was taken with, but an infrared camera could show it (See Thermography).
>
>Observation and use
>Main article: Thermal radiation
>
>In practice, virtually all solid or liquid substances start to glow around 798 K (525 °C) (977 °F), with a mildly dull red color, whether or not a chemical reaction takes place that produces light as a result of an exothermic process. This limit is called the Draper point. The incandescence does not vanish below that temperature, but it is too weak in the visible spectrum to be perceivable.
>At higher temperatures, the substance becomes brighter and its color changes from red towards white and finally blue.
>
>Incandescence is exploited in incandescent light bulbs, in which a filament is heated to a temperature at which a fraction of the radiation falls in the visible spectrum. The majority of the radiation however, is emitted in the infrared part of the spectrum, rendering incandescent lights relatively inefficient as a light source.[3] If the filament could be made hotter, efficiency would increase; however, there are currently no materials able to withstand such temperatures which would be appropriate for use in lamps.
>
>More efficient light sources, such as fluorescent lamps and LEDs, do not function by incandescence.
>Sunlight is the incandescence of the "white hot" surface of the sun.
>

>Incandescence
**Recall from #FreeEnergy Part 2 the Electromagnetic Spectrum. **
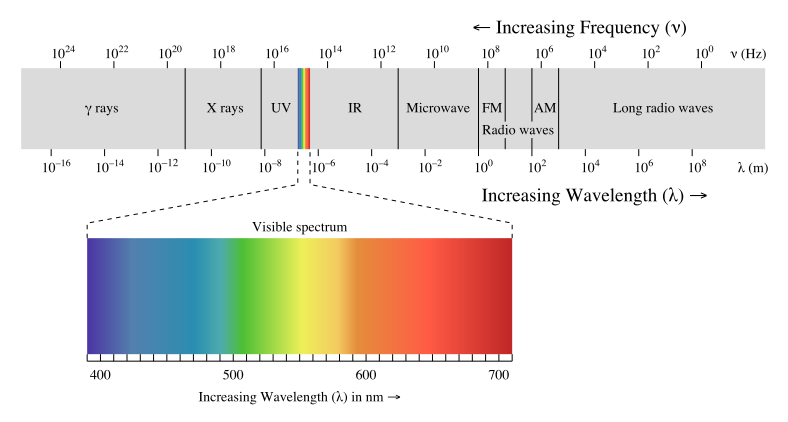
The Infrared part of the spectrum has less energy (hence less frequency/higher wavelength) than visible light.
https://en.wikipedia.org/wiki/Thermal_radiation
Retrieved: 9 November 2017
Archive: https://archive.is/8pdMX
Thermal radiation
>Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation. When the temperature of a body is greater than absolute zero, inter-atomic collisions cause the kinetic energy of the atoms or molecules to change. This results in charge-acceleration and/or dipole oscillation which produces electromagnetic radiation, and the wide spectrum of radiation reflects the wide spectrum of energies and accelerations that occur even at a single temperature.
>
>Examples of thermal radiation include the visible light and infrared light emitted by an incandescent light bulb, the infrared radiation emitted by animals that is detectable with an infrared camera, and the cosmic microwave background radiation. Thermal radiation is different from thermal convection and thermal conduction—a person near a raging bonfire feels radiant heating from the fire, even if the surrounding air is very cold.
>
>Sunlight is part of thermal radiation generated by the hot plasma of the Sun. The Earth also emits thermal radiation, but at a much lower intensity and different spectral distribution (infrared rather than visible) because it is cooler. The Earth's absorption of solar radiation, followed by its outgoing thermal radiation, have been held to be the two most important processes that determine the temperature and climate of the Earth in most climate models. However, radiant-heat trapping by freely convective gases has never been demonstrated experimentally[1].
>
>If a radiation-emitting object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation.[2] Planck's law describes the spectrum of blackbody radiation, which depends only on the object's temperature. Wien's displacement law determines the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the radiant intensity.[3]
>
>Thermal radiation is one of the fundamental mechanisms of heat transfer.
**MES Note:** More on Blackbody Radiation further below.
https://en.wikipedia.org/wiki/Light-emitting_diode
Retrieved: 6 November 2017
Archive: https://archive.is/oEDvS
Light-emitting diode
>A light-emitting diode (LED) is a two-lead semiconductor light source. It is a p–n junction diode that emits light when activated.[5] When a suitable voltage is applied to the leads, electrons are able to recombine with electron holes within the device, releasing energy in the form of photons. This effect is called electroluminescence, and the color of the light (corresponding to the energy of the photon) is determined by the energy band gap of the semiconductor. LEDs are typically small (less than 1 mm2) and integrated optical components may be used to shape the radiation pattern.[6]
>

>Blue, green, and red LEDs in 5 mm diffused case
>
>Parts of a conventional LED. The flat bottom surfaces of the anvil and post embedded inside the epoxy act as anchors, to prevent the conductors from being forcefully pulled out via mechanical strain or vibration.
https://en.wikipedia.org/wiki/Lead_(electronics)
Retrieved: 6 November 2017
Archive: https://archive.is/hAQ1k
Lead (electronics)
>In electronics, a lead is an electrical connection consisting of a length of wire or metal pad (SMD) that comes from a device. Leads are used for physical support, to transfer power, to probe circuits (see multimeter), to transmit information, and sometimes as a heatsink. The tiny leads coming off through-hole components are also often called pins.
>
>Many electrical components such as capacitors, resistors, and inductors have only two leads where some integrated circuits (ICs) can have several hundred leads to more than a thousand for the largest BGA devices.
>
>Several kinds of lead wires. A lead wire is a metal wire connected from the electric pole of an electronics part or an electronic component. The lead wire is a coated copper wire, a tinned copper wire or another electrically conductive wire used to connect two locations electrically.
https://en.wikipedia.org/wiki/P%E2%80%93n_junction
Retrieved: 6 November 2017
Archive: https://archive.is/YpQXO
p-n junction
>A p–n junction is a boundary or interface between two types of semiconductor material, p-type and n-type, inside a single crystal of semiconductor. The "p" (positive) side contains an excess of holes, while the "n" (negative) side contains an excess of electrons.
>
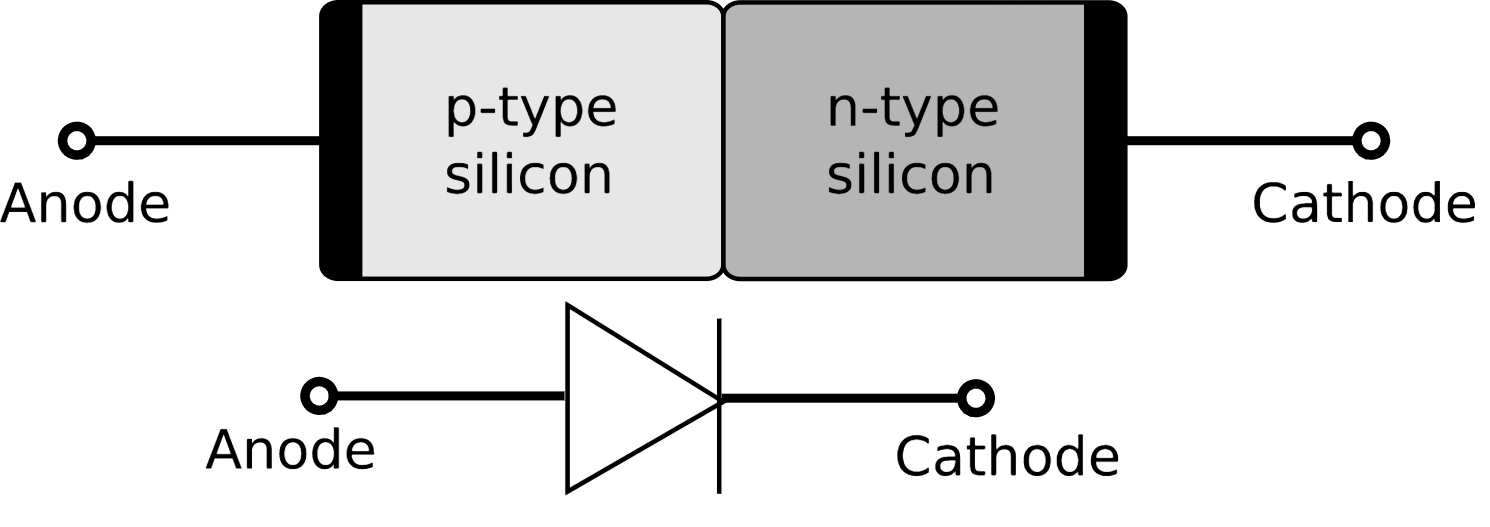
>A p–n junction. The circuit symbol is shown: the triangle corresponds to the p side.
https://en.wikipedia.org/wiki/Electroluminescence
Retrieved: 6 November 2017
Archive: https://archive.is/UGpM1
Electroluminescence
>Electroluminescence (EL) is an optical phenomenon and electrical phenomenon in which a material emits light in response to the passage of an electric current or to a strong electric field. This is distinct from black body light emission resulting from heat (incandescence), from a chemical reaction (chemiluminescence), sound (sonoluminescence), or other mechanical action (mechanoluminescence).
>
>Mechanism
>
>Electroluminescence is the result of radiative recombination of electrons and holes in a material, usually a semiconductor. The excited electrons release their energy as photons - light. Prior to recombination, electrons and holes may be separated either by doping the material to form a p-n junction (in semiconductor electroluminescent devices such as light-emitting diodes) or through excitation by impact of high-energy electrons accelerated by a strong electric field (as with the phosphors in electroluminescent displays).
>
>…
>

>The world's first electroluminescent billboard campaign, Canada, Winter 2005
>
>…
>

>1966 Dodge Charger instrument panel with electroluminescent lighting. Chrysler first introduced cars with EL panel lighting in its 1960 model year.
https://en.wikipedia.org/wiki/Black_body
Retrieved: 6 November 2017
Archive: https://archive.is/ienLb
Black body
>A black body is an idealized physical body that absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence. A white body is one with a "rough surface [that] reflects all incident rays completely and uniformly in all directions."[1]
>
>A black body in thermal equilibrium (that is, at a constant temperature) emits electromagnetic radiation called black-body radiation.
https://en.wikipedia.org/wiki/Black-body_radiation
Retrieved: 6 November 2017
Archive: https://archive.is/lmmNg
Black-body radiation
>Black-body radiation is the thermal electromagnetic radiation within or surrounding a body in thermodynamic equilibrium with its environment, or emitted by a black body (an opaque and non-reflective body). It has a specific spectrum and intensity that depends only on the body's temperature, which is assumed for the sake of calculations and theory to be uniform and constant.[1][2][3][4]
>
>>https://en.wikipedia.org/wiki/Thermodynamic_equilibrium
>>
>>Retrieved: 7 November 2017
>>Archive: https://archive.is/L2Por
>>
>>Thermodynamic equilibrium
>>
>>>In thermodynamic equilibrium there are no net macroscopic flows of matter or of energy, either within a system or between systems.
>
>The thermal radiation spontaneously emitted by many ordinary objects can be approximated as black-body radiation. A perfectly insulated enclosure that is in thermal equilibrium internally contains black-body radiation and will emit it through a hole made in its wall, provided the hole is small enough to have negligible effect upon the equilibrium.
>
>A black-body at room temperature appears black, as most of the energy it radiates is infra-red and cannot be perceived by the human eye. Because the human eye cannot perceive color at very low light intensities, a black body, viewed in the dark at the lowest just faintly visible temperature, subjectively appears grey (but only because the human eye is sensitive only to black and white at very low intensities - in reality, the frequency of the light in the visible range would still be red, although the intensity would be too low to discern as red), even though its objective physical spectrum peaks in the infrared range.[5] When it becomes a little hotter, it appears dull red. As its temperature increases further it eventually becomes blue-white.
>
>Although planets and stars are neither in thermal equilibrium with their surroundings nor perfect black bodies, black-body radiation is used as a first approximation for the energy they emit.[6] Black holes are near-perfect black bodies, in the sense that they absorb all the radiation that falls on them. It has been proposed that they emit black-body radiation (called Hawking radiation), with a temperature that depends on the mass of the black hole.[7]
>
>…
>
>Explanation
>
>All normal (baryonic) matter emits electromagnetic radiation when it has a temperature above absolute zero. The radiation represents a conversion of a body's thermal energy into electromagnetic energy, and is therefore called thermal radiation. It is a spontaneous process of radiative distribution of entropy.
>
>Conversely all normal matter absorbs electromagnetic radiation to some degree. An object that absorbs all radiation falling on it, at all wavelengths, is called a black body. When a black body is at a uniform temperature, its emission has a characteristic frequency distribution that depends on the temperature. Its emission is called black-body radiation.
>
>Color of a black body from 800 K to 12200 K. This range of colors approximates the range of colors of stars of different temperatures, as seen or photographed in the night sky.
>
>…
>
>Human-body emission
>
>The human body radiates energy as infrared light. The net power radiated is the difference between the power emitted and the power absorbed:
>
>P<sub>net</sub> = P<sub>emit</sub> - P<sub>absorb</sub>
>


>Such of a person's energy is radiated away in the form of infrared light. Some materials are transparent in the infrared, but opaque to visible light, as is the plastic bag in this infrared image (bottom). Other materials are transparent to visible light, but opaque or reflective in the infrared, noticeable by the darkness of the man's glasses.
https://en.wikipedia.org/wiki/Fluorescence
Retrieved: 6 November 2017
Archive: https://archive.is/3EfKD
Fluorescence
>Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. The most striking example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, while the emitted light is in the visible region, which gives the fluorescent substance a distinct color that can only be seen when exposed to UV light. Fluorescent materials cease to glow immediately when the radiation source stops, unlike phosphorescence, where it continues to emit light for some time after.
>

>Fluorescent minerals emit visible light when exposed to ultraviolet light
>
>Biofluorescent marine organisms
>

>Willemite and calcite in UV light
>
>…
>
>Photochemistry
>
>Fluorescence occurs when an orbital electron of a molecule, atom, or nanostructure, relaxes to its ground state by emitting a photon from an excited singlet state…
https://en.wikipedia.org/wiki/Ground_state
Retrieved: 6 November 2017
Archive: https://archive.is/ne1oV
Ground state
>The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In the quantum field theory, the ground state is usually called the vacuum state or the vacuum.
>
>Energy levels for an electron in an atom: ground state and excited states. After absorbing energy, an electron may jump from the ground state to a higher energy excited state.
https://en.wikipedia.org/wiki/Singlet_state
Retrieved: 6 November 2017
Archive: https://archive.is/S8jSr
Singlet state
>In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term singlet originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s = 0.
https://en.wikipedia.org/wiki/Electron_pair
Retrieved: 6 November 2017
Archive: https://archive.is/tcgoJ
Electron pair
>In chemistry, an electron pair or a Lewis pair consists of two electrons that occupy the same orbital but have opposite spins. The electron pair concept was introduced in a 1916 paper of Gilbert N. Lewis.[1]
>
>Because electrons are fermions, the Pauli exclusion principle forbids these particles from having exactly the same quantum numbers. Therefore, the only way to occupy the same orbital, i.e. have the same orbital quantum numbers, is to differ in the spin quantum number. This limits the number of electrons in the same orbital to exactly two.
>
>The pairing of spins is often energetically favorable and electron pairs therefore play a very large role in chemistry. They can form a chemical bond between two atoms, or they can occur as a lone pair of valence electrons. They also fill the core levels of an atom.
**MES Note:** I will be covering electrons and electron orbitals in much more detail in later parts so stay tuned!
https://en.wikipedia.org/wiki/Doping_(semiconductor)
Retrieved: 6 November 2017
Archive: https://archive.is/pmNY7
Doping (semiconductor)
>In semiconductor production, doping is the intentional introduction of impurities into an intrinsic semiconductor for the purpose of modulating its electrical properties.
https://en.wikipedia.org/wiki/Intrinsic_semiconductor
Retrieved: 6 November 2017
Archive: https://archive.is/RPomc
Intrinsic semiconductor
>An intrinsic semiconductor, also called an undoped semiconductor or i-type semiconductor, is a pure semiconductor without any significant dopant species present. The number of charge carriers is therefore determined by the properties of the material itself instead of the amount of impurities. In intrinsic semiconductors the number of excited electrons and the number of holes are equal: n = p.
https://en.wikipedia.org/wiki/Semiconductor
Retrieved: 6 November 2017
Archive: https://archive.is/wvbNf
Semiconductor
>A semiconductor material has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Their resistance decreases as their temperature increases, which is behavior opposite to that of a metal. Their conducting properties may be altered in useful ways by the deliberate, controlled introduction of impurities ("doping") into the crystal structure. Where two differently-doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers which include electrons, ions and electron holes at these junctions is the basis of diodes, transistors and all modern electronics.
https://en.wikipedia.org/wiki/Insulator_(electricity)
Retrieved: 6 November 2017
Archive: https://archive.is/FpO0y
Insulator (electricity)
>An electrical insulator is a material whose internal electric charges do not flow freely; very little electric current will flow through it under the influence of an electric field. This contrasts with other materials, semiconductors and conductors, which conduct electric current more easily. The property that distinguishes an insulator is its resistivity; insulators have higher resistivity than semiconductors or conductors.
>
>A perfect insulator does not exist, because even insulators contain small numbers of mobile charges (charge carriers) which can carry current. In addition, all insulators become electrically conductive when a sufficiently large voltage is applied that the electric field tears electrons away from the atoms. This is known as the breakdown voltage of an insulator. Some materials such as glass, paper and Teflon, which have high resistivity, are very good electrical insulators. A much larger class of materials, even though they may have lower bulk resistivity, are still good enough to prevent significant current from flowing at normally used voltages, and thus are employed as insulation for electrical wiring and cables. Examples include rubber-like polymers and most plastics which can be thermoset or thermoplastic in nature.
https://en.wikipedia.org/wiki/Superconductivity
Retrieved: 7 November 2017
Archive: https://archive.is/JW9Pc
Superconductivity
>Superconductivity is a phenomenon of exactly zero electrical resistance and expulsion of magnetic flux fields occurring in certain materials, called superconductors, when cooled below a characteristic critical temperature. It was discovered by Dutch physicist Heike Kamerlingh Onnes on April 8, 1911, in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum mechanical phenomenon. It is characterized by the Meissner effect, the complete ejection of magnetic field lines from the interior of the superconductor as it transitions into the superconducting state. The occurrence of the Meissner effect indicates that superconductivity cannot be understood simply as the idealization of perfect conductivity in classical physics.
>
>The electrical resistance of a metallic conductor decreases gradually as temperature is lowered. In ordinary conductors, such as copper or silver, this decrease is limited by impurities and other defects. Even near absolute zero, a real sample of a normal conductor shows some resistance. In a superconductor; the resistance drops abruptly to zero when the material is cooled below its critical temperature. An electric current through a loop of superconducting wire can persist indefinitely with no power source.[1][2][3][4]
>
>…
>

>A high-temperature superconductor levitating above a magnet
>
>…
>
>By critical temperature
>
>A superconductor is generally considered high-temperature if it reaches a superconducting state when cooled using liquid nitrogen – that is, at only T<sub>c</sub> > 77 K) – or low-temperature if more aggressive cooling techniques are required to reach its critical temperature.
>
>>**MES Note:** 77 Kelvin is -196.15 Celsius or -321.07 Fahrenheit, so still pretty cold ;)
https://en.wikipedia.org/wiki/Transistor
Retrieved: 6 November 2017
Archive: https://archive.is/3EfKD
Transistor
>A transistor is a semiconductor device used to amplify or switch electronic signals and electrical power.
>
>It is composed of semiconductor material usually with at least three terminals for connection to an external circuit. A voltage or current applied to one pair of the transistor's terminals controls the current through another pair of terminals. Because the controlled (output) power can be higher than the controlling (input) power, a transistor can amplify a signal.
>

>Assorted discrete transistors. Packages in order from top to bottom: TO-3, TO-126, TO-92, SOT-23.
>
>…
>
>Importance
>
>The transistor is the key active component in practically all modern electronics. Many consider it to be one of the greatest inventions of the 20th century.[31] Its importance in today's society rests on its ability to be mass-produced using a highly automated process (semiconductor device fabrication) that achieves astonishingly low per-transistor costs. The invention of the first transistor at Bell Labs was named an IEEE Milestone in 2009.[32]
>
>…
>
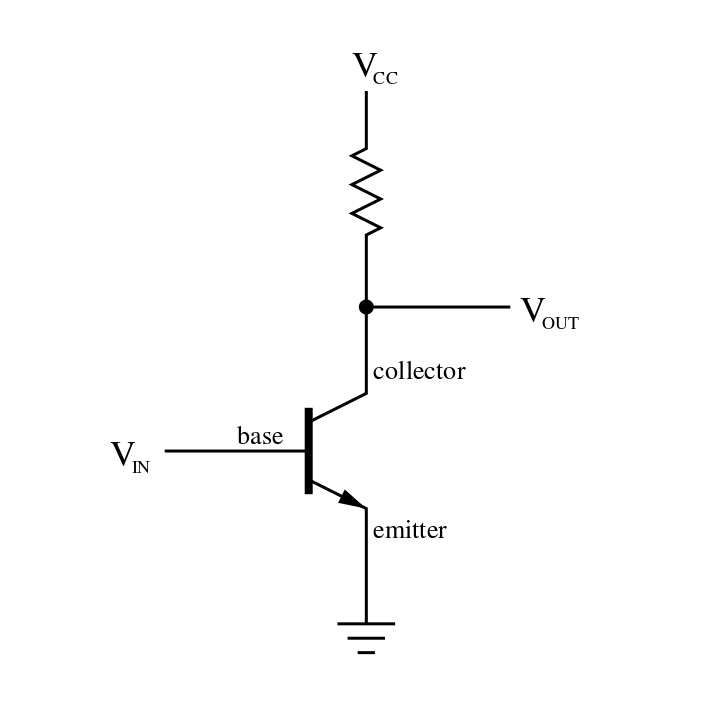
>A simple circuit diagram to show the labels of a n–p–n bipolar transistor.
**Let's briefly look at Condensed Matter Physics and Solid-State Physics:**
https://en.wikipedia.org/wiki/Condensed_matter_physics
Retrieved: 7 November 2017
Archive: https://archive.is/jSCxR
Condensed matter physics
>Condensed matter physics is a branch of physics that deals with the physical properties of condensed phases of matter,[1] where particles adhere to each other. Condensed matter physicists seek to understand the behavior of these phases by using physical laws. In particular, they include the laws of quantum mechanics, electromagnetism and statistical mechanics.
>
>The most familiar condensed phases are solids and liquids while more exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on crystal lattices of atoms, and the Bose–Einstein condensate found in ultracold atomic systems. The study of condensed matter physics involves measuring various material properties via experimental probes along with using methods of theoretical physics to develop mathematical models that help in understanding physical behavior.
>
>The diversity of systems and phenomena available for study makes condensed matter physics the most active field of contemporary physics: one third of all American physicists self-identify as condensed matter physicists,[2] and the Division of Condensed Matter Physics is the largest division at the American Physical Society.[3] The field overlaps with chemistry, materials science, and nanotechnology, and relates closely to atomic physics and biophysics. The theoretical physics of condensed matter shares important concepts and methods with that of particle physics and nuclear physics.[4]
>
>A variety of topics in physics such as crystallography, metallurgy, elasticity, magnetism, etc., were treated as distinct areas until the 1940s, when they were grouped together as solid state physics.
---
https://en.wikipedia.org/wiki/Solid-state_physics
Retrieved: 6 November 2017
Archive: https://archive.is/vPtSO
Solid-state physics
>Solid-state physics is the study of rigid matter, or solids, through methods such as quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic-scale properties. Thus, solid-state physics forms a theoretical basis of materials science. It also has direct applications, for example in the technology of transistors and semiconductors.
>
>…
>
>Background
>
>Solid materials are formed from densely packed atoms, which interact intensely. These interactions produce the mechanical (e.g. hardness and elasticity), thermal, electrical, magnetic and optical properties of solids. Depending on the material involved and the conditions in which it was formed, the atoms may be arranged in a regular, geometric pattern (crystalline solids, which include metals and ordinary water ice) or irregularly (an amorphous solid such as common window glass).
>
>The bulk of solid-state physics, as a general theory, is focused on crystals. Primarily, this is because the periodicity of atoms in a crystal — its defining characteristic — facilitates mathematical modeling. Likewise, crystalline materials often have electrical, magnetic, optical, or mechanical properties that can be exploited for engineering purposes.
>

>An example of a simple cubic lattice
https://en.wikipedia.org/wiki/Crystal
Retrieved: 7 November 2017
Archive: https://archive.is/DzjPE
Crystal
>A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions.[1][2] In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification.
>
>The word crystal derives from the Ancient Greek word ???sta???? (krustallos), meaning both "ice" and "rock crystal",[3] from ????? (kruos), "icy cold, frost".[4][5]
>
>Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Examples of polycrystals include most metals, rocks, ceramics, and ice. A third category of solids is amorphous solids, where the atoms have no periodic structure whatsoever. Examples of amorphous solids include glass, wax, and many plastics.
>
>Crystals are often used in pseudoscientific practices such as crystal therapy, and, along with gemstones, are sometimes associated with spellwork in Wiccan beliefs and related religious movements.[6][7][8]
>

>A crystal of amethyst quartz
>
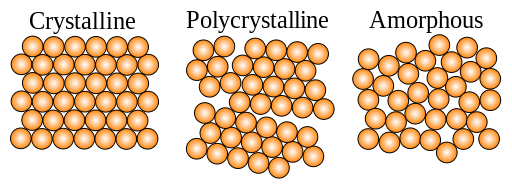
>Microscopically, a single crystal has atoms in a near-perfect periodic arrangement; a polycrystal is composed of many microscopic crystals (called "crystallites" or "grains"); and an amorphous solid (such as glass) has no periodic arrangement even microscopically.
**Back to the Atom Wikipedia Page:**
>Discovery of isotopes
>
>While experimenting with the products of radioactive decay, in 1913 radiochemist Frederick Soddy discovered that there appeared to be more than one type of atom at each position on the periodic table.[11] The term isotope was coined by Margaret Todd as a suitable name for different atoms that belong to the same element. J.J. Thomson created a technique for separating atom types through his work on ionized gases, which subsequently led to the discovery of stable isotopes.[12]
>
>Bohr model
>Main article: Bohr model
>
>In 1913 the physicist Niels Bohr proposed a model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes of energy corresponding to absorption or radiation of a photon.[13] This quantization was used to explain why the electrons orbits are stable (given that normally, charges in acceleration, including circular motion, lose kinetic energy which is emitted as electromagnetic radiation, see synchrotron radiation) and why elements absorb and emit electromagnetic radiation in discrete spectra.[14]
>
>Later in the same year Henry Moseley provided additional experimental evidence in favor of Niels Bohr's theory. These results refined Ernest Rutherford's and Antonius Van den Broek's model, which proposed that the atom contains in its nucleus a number of positive nuclear charges that is equal to its (atomic) number in the periodic table. Until these experiments, atomic number was not known to be a physical and experimental quantity. That it is equal to the atomic nuclear charge remains the accepted atomic model today.[15]
Link to GIF: [https://commons.wikimedia.org/wiki/File:Bohr_atom_animation_2.gif](https://commons.wikimedia.org/wiki/File:Bohr_atom_animation_2.gif)
>>Retrieved: 3 November 2017
>>Archive: https://archive.is/EJoWz
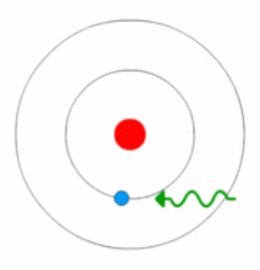
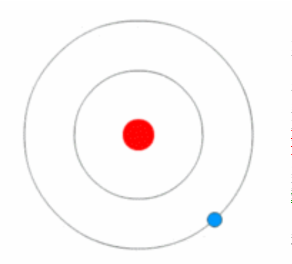
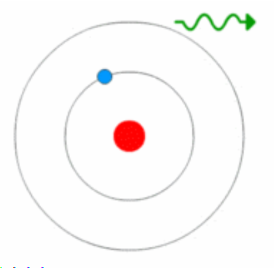
>The Bohr model of the atom, with an electron making instantaneous "quantum leaps" from one orbit to another. This model is obsolete.
>
>Chemical bonding explained
>
>Chemical bonds between atoms were now explained, by Gilbert Newton Lewis in 1916, as the interactions between their constituent electrons.[16] As the chemical properties of the elements were known to largely repeat themselves according to the periodic law,[17] in 1919 the American chemist Irving Langmuir suggested that this could be explained if the electrons in an atom were connected or clustered in some manner. Groups of electrons were thought to occupy a set of electron shells about the nucleus.[18]
>
>…
>
>Structure
>
>Subatomic particles
>Main article: Subatomic particle
>
>Though the word atom originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various subatomic particles. The constituent particles of an atom are the electron, the proton and the neutron; all three are fermions. However, the hydrogen-1 atom has no neutrons and the hydron ion has no electrons.
https://en.wikipedia.org/wiki/Hydron_(chemistry)
Retrieved: 2 November 2017
Archive: https://archive.is/CyHiq
Hydron (chemistry)
>In chemistry, a hydron is the general name for a cationic form of atomic hydrogen, represented with the symbol H+. However, this term is avoided and instead "proton" is used, which strictly speaking refers to the cation of protium, the most common isotope of hydrogen. The term "hydron" includes cations of hydrogen regardless of their isotopic composition: thus it refers collectively to protons (<sup>1</sup>H<sup>+</sup>) for the protium isotope, deuterons (<sup>2</sup>H<sup>+</sup> or D<sup>+</sup>) for the deuterium isotope, and tritons (<sup>3</sup>H<sup>+</sup> or T<sup>+</sup>) for the tritium isotope. Unlike other ions, the hydron consists only of a bare atomic nucleus.
>
>The negatively charged counterpart of the hydron is the hydride anion, H<sup>-</sup>.
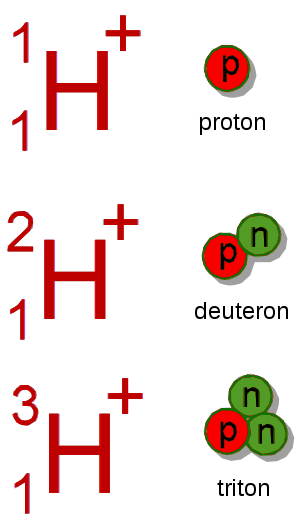
https://en.wikipedia.org/wiki/Hydrogen_anion
Retrieved: 2 October 2017
Archive: https://archive.is/dNq5b
Hydrogen anion
>The hydrogen anion is a negative ion of hydrogen, H<sup>-</sup>. The hydrogen anion is an important constituent of the atmosphere of stars, such as the Sun. In chemistry, this ion is called hydride. The ion has two electrons bound by the electromagnetic force to a nucleus containing one proton.
>
>Occurrence
>
>The hydrogen anion is an important species in the photosphere of the Sun. It absorbs energies in the range 0.75–4.0 eV, which ranges from the infrared into the visible spectrum (Rau 1999, Srinivasan 1999). It also occurs in the Earth's ionosphere (Rau 1999), and can be produced in particle accelerators.
>
>Its existence was first proven theoretically by Hans Bethe in 1929 (Bethe 1929). H- is unusual because, in its free form, it has no bound excited states, as was finally proven in 1977 (Hill 1977). It has been studied experimentally using particle accelerators (Bryant 1977).
>
>In chemistry, the hydride anion is hydrogen that has the formal oxidation state -1.
>
>The term hydride is probably most often used to describe compounds of hydrogen with other elements in which the hydrogen is in the formal -1 oxidation state.
https://en.wikipedia.org/wiki/Oxidation_state
Retrieved: 2 November 2017
Archive: https://archive.is/mSE64
Oxidation state
>The oxidation state, sometimes referred to as oxidation number, is an indicator of the degree of oxidation (loss of electrons) of an atom in a chemical compound. Conceptually, the oxidation state, which may be positive, negative or zero, is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic, with no covalent component. This is never exactly true for real bonds.
https://en.wikipedia.org/wiki/Covalent_bond
Retrieved: 3 November 2017
Archive: https://archive.is/VBZRu
Covalent bond
>A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.[1][better source needed] For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full outer shell, corresponding to a stable electronic configuration.
>
>Covalent bonding includes many kinds of interactions, including s-bonding, p-bonding, metal-to-metal bonding, agostic interactions, bent bonds, and three-center two-electron bonds.[2][3] The term covalent bond dates from 1939.[4] The prefix co- means jointly, associated in action, partnered to a lesser degree, etc.; thus a "co-valent bond", in essence, means that the atoms share "valence", such as is discussed in valence bond theory.
>
>In the molecule H2, the hydrogen atoms share the two electrons via covalent bonding.[5] Covalency is greatest between atoms of similar electronegativities. Thus, covalent bonding does not necessarily require that the two atoms be of the same elements, only that they be of comparable electronegativity. Covalent bonding that entails sharing of electrons over more than two atoms is said to be delocalized.
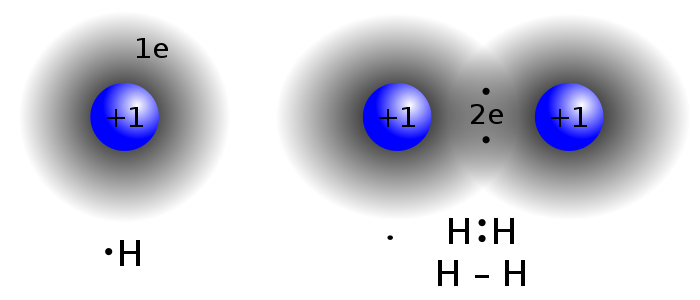
>A covalent bond forming H2 (right) where two hydrogen atoms share the two electrons
>
>…
>
>One- and three-electron bonds
>
>Bonds with one or three electrons can be found in radical species, which have an odd number of electrons. The simplest example of a 1-electron bond is found in the dihydrogen cation, H2+.
>
>…
>
>The simplest example of three-electron bonding can be found in the helium dimer cation, He2+. It is considered a "half bond" because it consists of only one shared electron (rather than two); in molecular orbital terms, the third electron is in an anti-bonding orbital which cancels out half of the bond formed by the other two electrons. Another example of a molecule containing a 3-electron bond, in addition to two 2-electron bonds, is nitric oxide, NO.
>
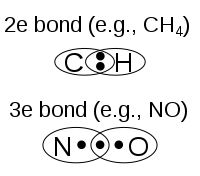
>Comparison of the electronic structure of the three-electron bond to the conventional covalent bond.[11]
https://en.wikipedia.org/wiki/Electronegativity
Retrieved: 3 November 2017
Archive: https://archive.is/daZMy
Electronegativity
>Electronegativity, symbol x, is a chemical property that describes the tendency of an atom to attract electrons (or electron density) towards itself.[1] An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it.
>
>The term "electronegativity" was introduced by Jöns Jacob Berzelius in 1811,[2] though the concept was known even before that and was studied by many chemists including Avogadro.[2] In spite of its long history, an accurate scale of electronegativity was not developed until 1932, when Linus Pauling proposed an electronegativity scale, which depends on bond energies, as a development of valence bond theory.[3] It has been shown to correlate with a number of other chemical properties. Electronegativity cannot be directly measured and must be calculated from other atomic or molecular properties. Several methods of calculation have been proposed, and although there may be small differences in the numerical values of the electronegativity, all methods show the same periodic trends between elements.
>
>The most commonly used method of calculation is that originally proposed by Linus Pauling. This gives a dimensionless quantity, commonly referred to as the Pauling scale (x<sup>r</sup>), on a relative scale running from around 0.7 to 3.98 (hydrogen = 2.20). When other methods of calculation are used, it is conventional (although not obligatory) to quote the results on a scale that covers the same range of numerical values: this is known as an electronegativity in Pauling units.
>
>Electrostatic potential map of a water molecule, where the oxygen atom has a more negative charge (red) than the positive (blue) hydrogen atoms
https://en.wikipedia.org/wiki/Ionic_compound
Retrieved: 3 November 2017
Archive: https://archive.is/0n52Y
Ionic compound
>In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. These can be simple ions such as the sodium (Na<sup>+</sup>) and chloride (Cl<sup>-</sup>) in sodium chloride, or polyatomic species such as the ammonium (NH<sub>4</sub><sup>+</sup>) and carbonate (CO<sub>3</sub><sup>2-</sup>) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network, usually in a crystalline structure.
>
>Ionic compounds containing hydrogen ions (H<sup>+</sup>) are classified as acids, and those containing basic ions hydroxide (OH<sup>-</sup>) or oxide (O<sup>2-</sup>) are classified as bases. Ionic compounds without these ions are also known as salts and can be formed by acid–base reactions. Ionic compounds can also be produced from their constituent ions by evaporation of their solvent, precipitation, freezing, a solid-state reaction, or the electron transfer reaction of reactive metals with reactive non-metals, such as halogen gases.
>
>Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids they are almost always electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.
>
>The crystal structure of sodium chloride, NaCl, a typical ionic compound. The purple spheres represent sodium cations, Na+, and the green spheres represent chloride anions, Cl<sup>-</sup>.
>
>…
>
>Bonding
>Main article: Ionic bonding
>
>Ions in ionic compounds are primarily held together by the electrostatic forces between the charge distribution of these bodies, and in particular the ionic bond resulting from the long-ranged Coulomb attraction between the net negative charge of the anions and net positive charge of the cations.[19] There is also a small additional attractive force from van der Waals interactions which contributes only around 1–2% of the cohesive energy for small ions.[20] When a pair of ions comes close enough for their outer electron shells (most simple ions have closed shells) to overlap, a short-ranged repulsive force occurs,[21] due to the Pauli exclusion principle.[22] The balance between these forces leads to a potential energy well with a minimum energy when the nuclei are separated by a specific equilibrium distance.[21]
>
>If the electronic structure of the two interacting bodies is affected by the presence of one another, covalent interactions (non-ionic) also contribute to the overall energy of the compound formed.[23] Ionic compounds are rarely purely ionic, i.e. held together only by electrostatic forces. The bonds between even the most electronegative/electropositive pairs such as those in caesium fluoride exhibit a small degree of covalency.[24][25]Conversely, covalent bonds between unlike atoms often exhibit some charge separation and can be considered to have a partial ionic character.[23]
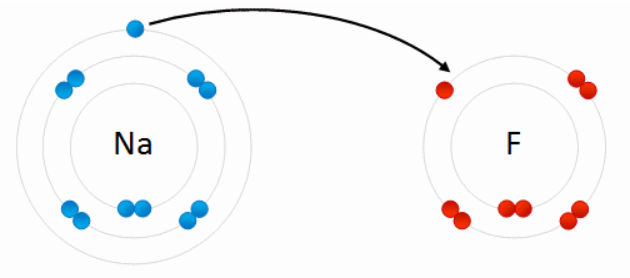
>A schematic electron shell diagram of sodium and fluorine atoms undergoing a redox reaction to form sodium fluoride. Sodium loses its outer electron to give it a stable electron configuration, and this electron enters the fluorine atom exothermically. The oppositely charged ions – typically a great many of them – are then attracted to each other to form a solid.
Link to GIF: [https://commons.wikimedia.org/wiki/File:NaF.gif](https://commons.wikimedia.org/wiki/File:NaF.gif)
>>
>>Retrieved: 3 November 2017
>>Archive: https://archive.is/kUPJo
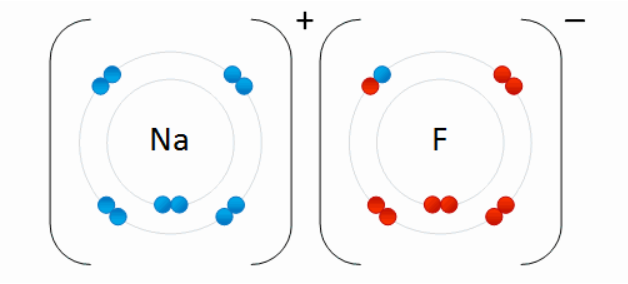
>>**MES Note:** Notice how the initially neutral <sup>11</sup>Na combines with the neutral <sup>9</sup>F to form a more stable ionic compound of the ions <sub>11</sub>Na<sup>+</sup> and <sub>9</sub>F<sup>-</sup>, because the less stable valence electron configurations drove the ionic bonding to form more stable valence electron configurations in the resulting ionic compound.
>
>…

>Halite, the mineral form of sodium chloride, forms when salty water evaporates leaving the ions behind.
>
>…
>
>Defects
>See also: crystallographic defect
>
>Within an ionic crystal, there will usually be some point defects, but to maintain electroneutrality, these defects come in pairs.[53] Frenkel defects consist of a cation vacancy paired with a cation interstitial and can be generated anywhere in the bulk of the crystal,[53] occurring most commonly in compounds with a low coordination number and cations that are much smaller than the anions.[54] Schottky defects consist of one vacancy of each type, and are generated at the surfaces of a crystal,[53] occurring most commonly in compounds with a high coordination number and when the anions and cations are of similar size.[54]
>
>Frenkel defect
>
>Schottky defect
https://en.wikipedia.org/wiki/Van_der_Waals_force
Retrieved: 3 November 2017
Archive: https://archive.is/WcKUu
Van der Waals force
>In physical chemistry, the van der Waals forces, named after Dutch scientist Johannes Diderik van der Waals, are distance-dependent interactions between atoms or molecules. Unlike ionic or covalent bonds, these attractions are not a result of any chemical electronic bond, and they are comparatively weak and more susceptible to being perturbed. Van der Waals forces quickly vanish at longer distances between interacting molecules.
>
>…
>
>The resulting van der Waals forces can be attractive or repulsive.[3] It is also sometimes used loosely as a synonym for the totality of intermolecular forces.[4] The term includes the force between permanent dipoles (Keesom force), the force between a permanent dipole and a corresponding induced dipole (Debye force), and the force between instantaneously induced dipoles (London dispersion force).
>

>Geckos can stick to walls and ceilings because of van der Waals forces.
https://en.wikipedia.org/wiki/Dipole#Molecular_dipoles
Retrieved: 3 November 2017
Archive: https://archive.is/jKLis
Dipole
>In electromagnetism, there are two kinds of dipoles:
>
>• An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some (usually small) distance. A permanent electric dipole is called an electret.
>• A magnetic dipole is a closed circulation of electric current. A simple example of this is a single loop of wire with some constant current through it.[1][2]
>
>…
>
>Molecular dipoles
>See also: chemical polarity
>
>Many molecules have such dipole moments due to non-uniform distributions of positive and negative charges on the various atoms. Such is the case with polar compounds like hydrogen fluoride (HF), where electron density is shared unequally between atoms. Therefore, a molecule's dipole is an electric dipole with an inherent electric field which should not be confused with a magnetic dipole which generates a magnetic field.
>
>The physical chemist Peter J. W. Debye was the first scientist to study molecular dipoles extensively, and, as a consequence, dipole moments are measured in units named debye in his honor.
>
>For molecules there are three types of dipoles:
>
>• Permanent dipoles: These occur when two atoms in a molecule have substantially different electronegativity: One atom attracts electrons more than another, becoming more negative, while the other atom becomes more positive. A molecule with a permanent dipole moment is called a polar molecule. See dipole–dipole attractions.
>• Instantaneous dipoles: These occur due to chance when electrons happen to be more concentrated in one place than another in a molecule, creating a temporary dipole. See instantaneous dipole.
>• Induced dipoles: These can occur when one molecule with a permanent dipole repels another molecule's electrons, inducing a dipole moment in that molecule. A molecule is polarized when it carries an induced dipole. See induced-dipole attraction.
This video is a very good illustration of the Van der Waals forces.
**Van der Waals forces are essentially forces due to uneven distribution of molecular charges, either temporary or permanently.**
[https://youtu.be/HGc9RFD7iSE](https://youtu.be/HGc9RFD7iSE)
Retrieved: 3 November 2017
Archive: https://archive.is/WMrz6
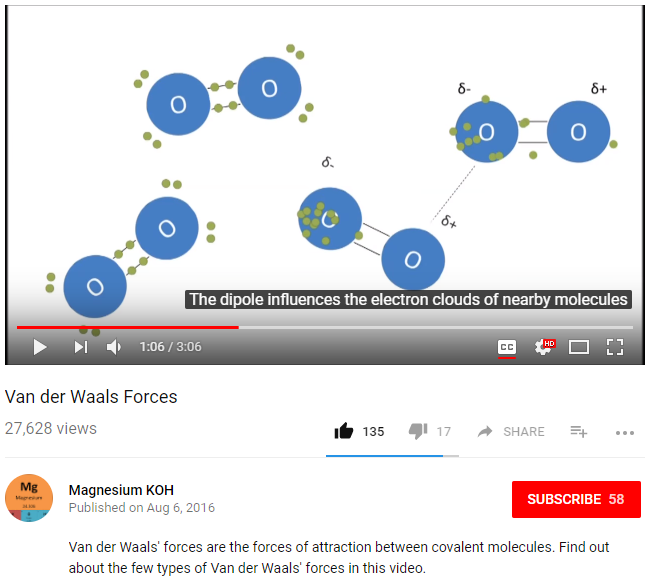
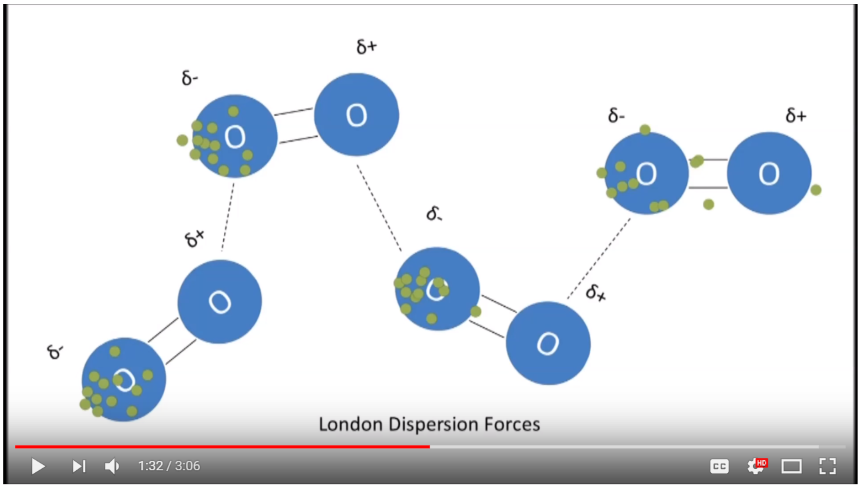
London Dispersion Forces are instantaneously generated dipoles from the random uneven distribution of electrons in an atom or molecule.
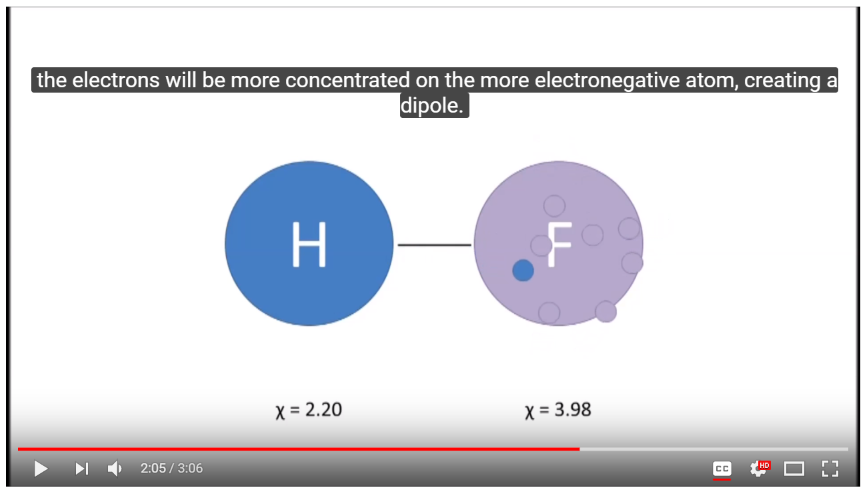
Molecules made up of atoms with dissimilar electronegativities create permanent dipoles, called Polar Molecules.
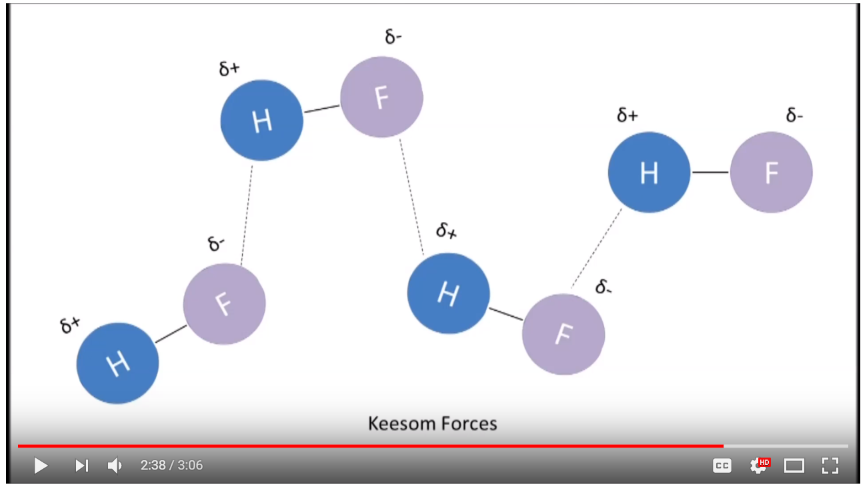
Keesom Forces are between Polar Molecules.
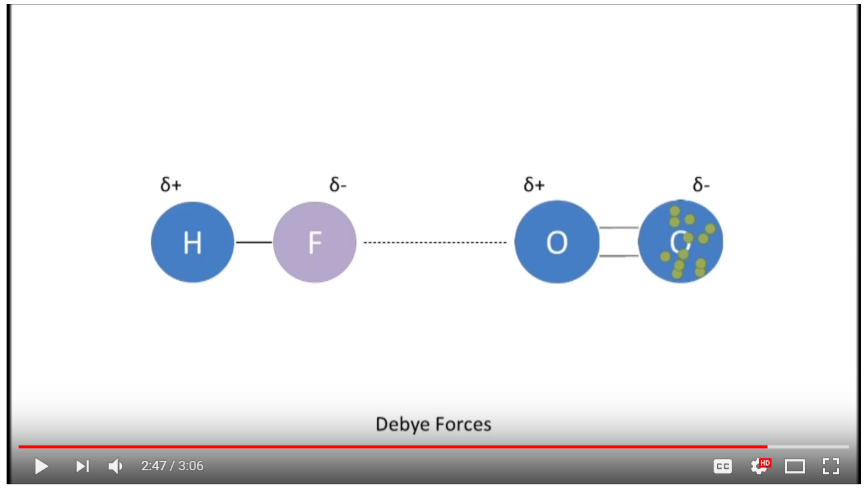
Debye Forces are forces between Polar Molecules and Molecules with induced Polarity from Polar Molecules.
https://en.wikipedia.org/wiki/Intermolecular_force
Retrieved: 7 November 2017
Archive: https://archive.is/P4khH
Intermolecular force
>Intermolecular forces (IMFs) are the forces which mediate interaction between molecules, including forces of attraction or repulsion which act between molecules and other types of neighboring particles, e.g., atoms or ions. Inter-molecular forces are weak relative to intramolecular forces – the forces which hold a molecule together.
>
>For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics.
>
>The investigation of inter-molecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level.
>
>…
>
>Attractive intermolecular forces are considered by the following types:
>
>• Ion-induced dipole forces
>• Ion-dipole forces
>• Hydrogen bonding
>• van der Waals forces – Keesom force, Debye force, and London dispersion force
>
>Van der Waals forces
>Main article: van der Waals force
>
>The van der Waals forces arise from interaction between uncharged atoms or molecules, leading not only to such phenomena as the cohesion of condensed phases and physical adsorption of gases, but also to a universal force of attraction between macroscopic bodies.[4]
https://en.wikipedia.org/wiki/Adsorption
Retrieved: 7 November 2017
Archive: https://archive.is/6LByv
Adsorption
>Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface.[1] This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid (the absorbate) is dissolved by or permeates a liquid or solid (the absorbent), respectively.[2] Adsorption is a surface-based process while absorption involves the whole volume of the material. The term sorption encompasses both processes, while desorption is the reverse of it. Adsorption is a surface phenomenon.
>
>Similar to surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are filled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the bonding depends on the details of the species involved, but the adsorption process is generally classified as physisorption (characteristic of weak van der Waals forces) or chemisorption (characteristic of covalent bonding). It may also occur due to electrostatic attraction.[4]
https://en.wikipedia.org/wiki/Intramolecular_force
Retrieved: 7 November 2017
Archive: https://archive.is/KNuh2
Intramolecular force
>An intramolecular force is any force that holds together the atoms making up a molecule or compound.[1] This includes all types of chemical bonds. They are usually stronger than intermolecular forces, which are present between atoms or molecules that are not bonded. Hydrogen bonds are an important example of a force that can be either intramolecular or intermolecular.
https://en.wikipedia.org/wiki/Hydrogen_bond
Retrieved: 7 November 2017
Archive: https://archive.is/SxkeS
Hydrogen bond
>A hydrogen bond is an electrostatic attraction between two polar groups that occurs when a hydrogen (H) atom covalently bound to a highly electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F) experiences the electrostatic field of another highly electronegative atom nearby.
>
>Hydrogen bonds can occur between molecules (intermolecular) or within different parts of a single molecule (intramolecular).[4] Depending on the nature of the donor and acceptor atoms which constitute the bond, their geometry, and environment, the energy of a hydrogen bond can vary between 1 and 40 kcal/mol [5]. This makes them somewhat stronger than a van der Waals interaction, and weaker than covalent or ionic bonds. This type of bond can occur in inorganic molecules such as water and in organic molecules like DNA and proteins.
>
>Intermolecular hydrogen bonding is responsible for the high boiling point of water (100 °C) compared to the other group 16 hydrides that have much weaker hydrogen bonds.[6]
https://en.wikipedia.org/wiki/Isotopes_of_hydrogen
Retrieved: 2 November 2017
Archive: https://archive.is/qKz5u
Isotopes of hydrogen
>Hydrogen (<sub>1</sub>H) has three naturally occurring isotopes, sometimes denoted <sup>1</sup>H, <sup>2</sup>H, and <sup>3</sup>H. The first two of these are stable while <sup>3</sup>H has a half-life of 12.32 years. All heavier isotopes are synthetic and have a half-life less than one zeptosecond (10<sup>-21</sup> second). Of these, <sup>5</sup>H is the most stable, and <sup>7</sup>H is the least.[2][3]
>
>Hydrogen is the only element whose isotopes have different names that are in common use today. The <sup>2</sup>H (or hydrogen-2) isotope is usually called deuterium, while the <sup>3</sup>H (or hydrogen-3) isotope is usually called tritium. The symbols D and T (instead of <sup>2</sup>H and <sup>3</sup>H) are sometimes used for deuterium and tritium. The IUPAC states in the 2005 Red Book that while the use of D and T is common, it is not preferred because it can cause problems in the alphabetic sorting of chemical formulas. The ordinary isotope of hydrogen, with no neutrons, is sometimes called "protium".
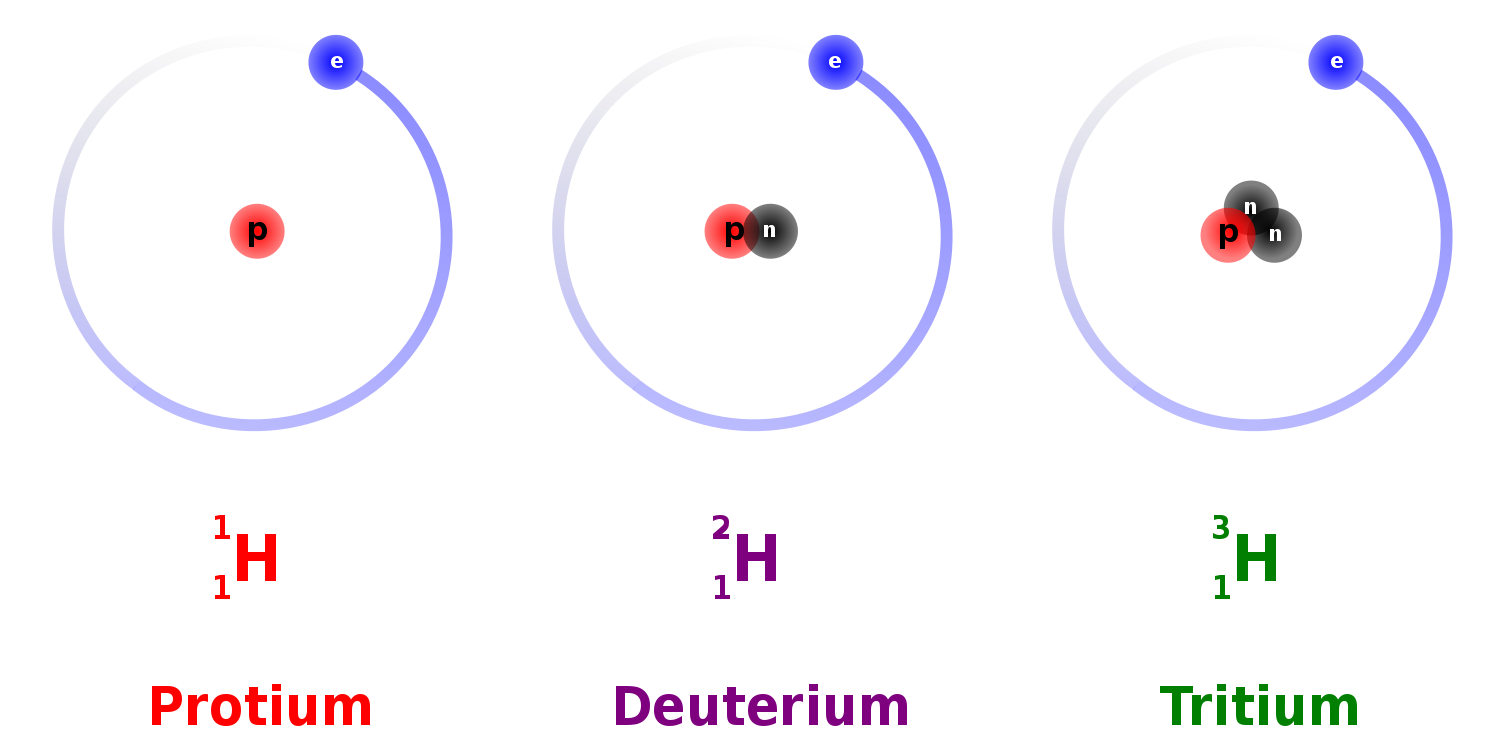
>The three most stable isotopes of hydrogen: protium (A = 1), deuterium (A = 2), and tritium (A = 3).
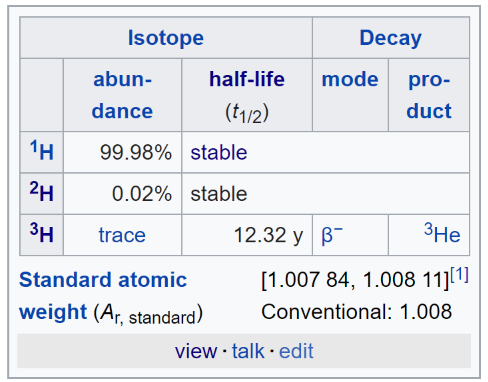
>Main isotopes of hydrogen
>
>…
>
>Hydrogen-2 (deuterium)
>For more details on this topic, see deuterium.
>
>…
>
>Deuterium is not radioactive, and does not represent a significant toxicity hazard. Water enriched in molecules that include deuterium instead of protium is called heavy water.
>
>Deuterium and its compounds are used as a non-radioactive label in chemical experiments and in solvents for 1H-NMR spectroscopy. Heavy water is used as a neutron moderator and coolant for nuclear reactors. Deuterium is also a potential fuel for commercial nuclear fusion.
>
>…
>
>Hydrogen-3 (tritium)
>For more details on this topic, see tritium.
>
>…
>
>Trace amounts of tritium occur naturally because of the interaction of cosmic rays with atmospheric gases. Tritium has also been released during nuclear weapons tests. It is used in thermonuclear fusion weapons, as a tracer in isotope geochemistry, and specialized in self-powered lighting devices.
>
>The most common method of producing tritium is by bombarding a natural isotope of lithium, lithium-6, with neutrons in a nuclear reactor.
>
>Tritium was once used routinely in chemical and biological labeling experiments as a radiolabel, which has become less common in recent times. D-T nuclear fusion uses tritium as its main reactant, along with deuterium, liberating energy through the loss of mass when the two nuclei collide and fuse at high temperatures.
https://en.wikipedia.org/wiki/Radioactive_tracer
Retrieved: 2 November 2017
Archive: https://archive.is/T13gl
Radioactive tracer
>A radioactive tracer, or radioactive label, is a chemical compound in which one or more atoms have been replaced by a radioisotope so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling is thus the radioactive form of isotopic labeling.
https://en.wikipedia.org/wiki/Radionuclide
Retrieved: 2 October 2017
Archive: https://archive.is/eqi88
Radionuclide
(Redirected from Radioisotope)
>A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is an atom that has excess nuclear energy, making it unstable. This excess energy can be either emitted from the nucleus as gamma radiation, or create and emit from the nucleus a new particle (alpha particle or beta particle), or transfer this excess energy to one of its electrons, causing that electron to be ejected as a conversion electron. During those processes, the radionuclide is said to undergo radioactive decay.[1] These emissions constitute ionizing radiation. The unstable nucleus is more stable following the emission, but will sometimes undergo further decay.
https://en.wikipedia.org/wiki/Ionizing_radiation
Retrieved: 2 October 2017
Archive: https://archive.is/lIWdZ
Ionizing radiation
>Ionizing radiation (ionising radiation) is radiation that carries enough energy to liberate electrons from atoms or molecules, thereby ionizing them. Ionizing radiation is made up of energetic subatomic particles, ions or atoms moving at high speeds (usually greater than 1% of the speed of light), and electromagnetic waves on the high-energy end of the electromagnetic spectrum.
>
>Gamma rays, X-rays, and the higher ultraviolet part of the electromagnetic spectrum are ionizing, whereas the lower ultraviolet part of the electromagnetic spectrum, and also the lower part of the spectrum below UV, including visible light (including nearly all types of laser light), infrared, microwaves, and radio waves are all considered non-ionizing radiation.

>Ionizing radiation hazard symbol
**Back to the Atom Wikipedia page:**
>Structure
>
>Subatomic particles
>Main article: Subatomic particle
>
>Though the word atom originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various subatomic particles. The constituent particles of an atom are the electron, the proton and the neutron; all three are fermions. However, the hydrogen-1 atom has no neutrons and the hydron ion has no electrons.
>
>The electron is by far the least massive of these particles at 9.11×10<sup>-31</sup> kg, with a negative electrical charge and a size that is too small to be measured using available techniques.[32] It is the lightest particle with a positive rest mass measured. Under ordinary conditions, electrons are bound to the positively charged nucleus by the attraction created from opposite electric charges. If an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively charged as a whole; a charged atom is called an ion. Electrons have been known since the late 19th century, mostly thanks to J.J. Thomson; see history of subatomic physics for details.
>
>Protons have a positive charge and a mass 1,836 times that of the electron, at 1.6726×10<sup>-27</sup> kg. The number of protons in an atom is called its atomic number. Ernest Rutherford (1919) observed that nitrogen under alpha-particle bombardment ejects what appeared to be hydrogen nuclei. By 1920 he had accepted that the hydrogen nucleus is a distinct particle within the atom and named it proton.
>
>Neutrons have no electrical charge and have a free mass of 1,839 times the mass of the electron,[33] or 1.6929×10<sup>-27</sup> kg, the heaviest of the three constituent particles, but it can be reduced by the nuclear binding energy. Neutrons and protons (collectively known as nucleons) have comparable dimensions—on the order of 2.5×10<sup>-15</sup> m—although the 'surface' of these particles is not sharply defined.[34] The neutron was discovered in 1932 by the English physicist James Chadwick.
>
>In the Standard Model of physics, electrons are truly elementary particles with no internal structure. However, both protons and neutrons are composite particles composed of elementary particles called quarks. There are two types of quarks in atoms, each having a fractional electric charge.
>
>Nucleus
>Main article: Atomic nucleus
>
>All the bound protons and neutrons in an atom make up a tiny atomic nucleus, and are collectively called nucleons. The radius of a nucleus is approximately equal to 1.07 A<sup>1/3</sup>3 fm, where A is the total number of nucleons.[37] This is much smaller than the radius of the atom, which is on the order of 10<sup>5</sup> fm. The nucleons are bound together by a short-ranged attractive potential called the residual strong force. At distances smaller than 2.5 fm this force is much more powerful than the electrostatic force that causes positively charged protons to repel each other.[38]
>
>…
>
>The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. Nuclear fusion occurs when multiple atomic particles join to form a heavier nucleus, such as through the energetic collision of two nuclei. For example, at the core of the Sun protons require energies of 3–10 keV to overcome their mutual repulsion—the coulomb barrier—and fuse together into a single nucleus.[41] Nuclear fission is the opposite process, causing a nucleus to split into two smaller nuclei—usually through radioactive decay. The nucleus can also be modified through bombardment by high energy subatomic particles or photons. If this modifies the number of protons in a nucleus, the atom changes to a different chemical element.[42][43]
>
>If the mass of the nucleus following a fusion reaction is less than the sum of the masses of the separate particles, then the difference between these two values can be emitted as a type of usable energy (such as a gamma ray, or the kinetic energy of a beta particle), as described by Albert Einstein's mass–energy equivalence formula, E = mc<sup>2</sup>, where m is the mass loss and c is the speed of light. This deficit is part of the binding energy of the new nucleus, and it is the non-recoverable loss of the energy that causes the fused particles to remain together in a state that requires this energy to separate.[44]
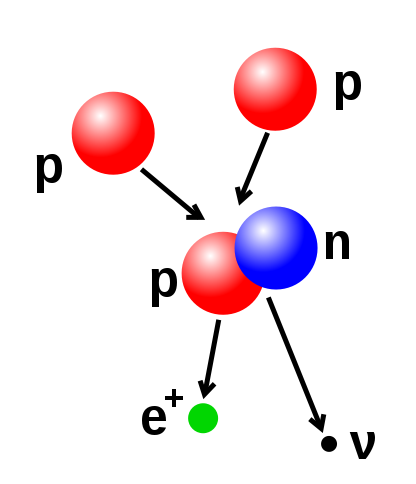
>Illustration of a nuclear fusion process that forms a deuterium nucleus, consisting of a proton and a neutron, from two protons. A positron (e<sup>+</sup>)—an antimatter electron—is emitted along with an electron neutrino.
>
>Electron cloud
>Main articles: Atomic orbital and Electron configuration
>
>The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means that an external source of energy is needed for the electron to escape. The closer an electron is to the nucleus, the greater the attractive force. Hence electrons bound near the center of the potential well require more energy to escape than those at greater separations.
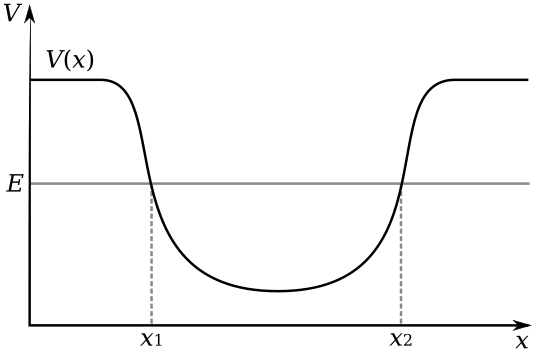
>A potential well, showing, according to classical mechanics, the minimum energy V(x) needed to reach each position x. Classically, a particle with energy E is constrained to a range of positions between x<sub>1</sub> and x<sub>2</sub>.
>
>Electrons, like other particles, have properties of both a particle and a wave. The electron cloud is a region inside the potential well where each electron forms a type of three-dimensional standing wave—a wave form that does not move relative to the nucleus. This behavior is defined by an atomic orbital, a mathematical function that characterises the probability that an electron appears to be at a particular location when its position is measured.[46] Only a discrete (or quantized) set of these orbitals exist around the nucleus, as other possible wave patterns rapidly decay into a more stable form.[47] Orbitals can have one or more ring or node structures, and differ from each other in size, shape and orientation.[48]
>
>Each atomic orbital corresponds to a particular energy level of the electron. The electron can change its state to a higher energy level by absorbing a photon with sufficient energy to boost it into the new quantum state. Likewise, through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for atomic spectral lines.[47]
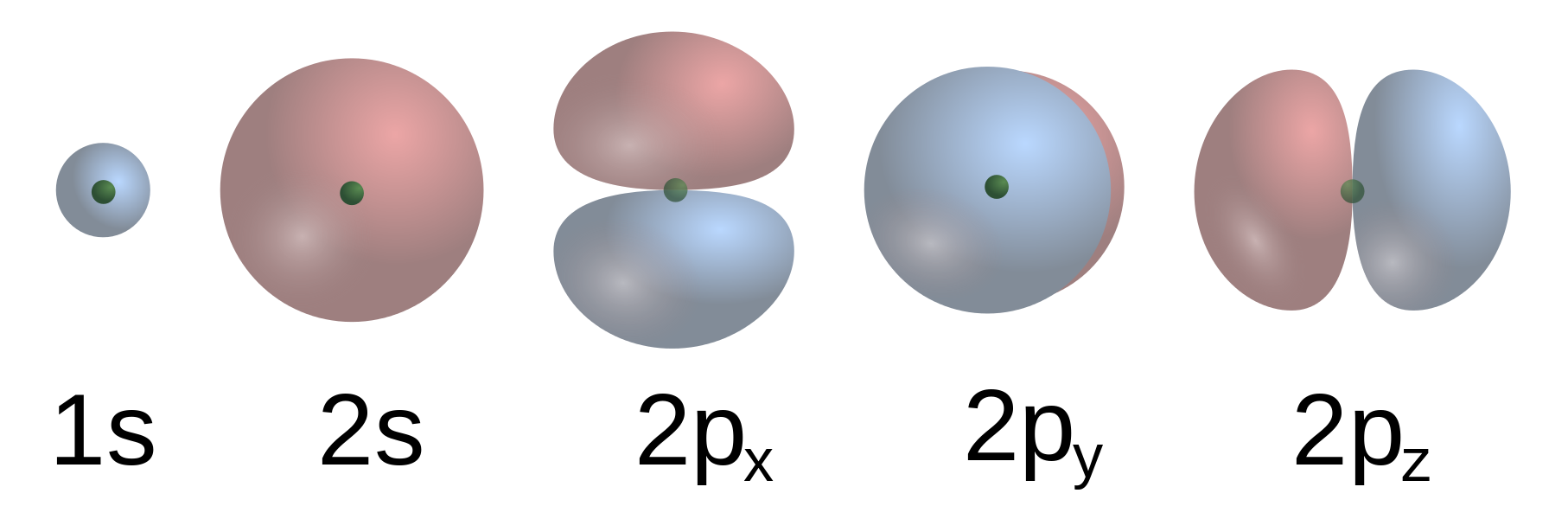
>Wave functions of the first five atomic orbitals. The three 2p orbitals each display a single angular node that has an orientation and a minimum at the center.
>
>The amount of energy needed to remove or add an electron—the electron binding energy—is far less than the binding energy of nucleons. For example, it requires only 13.6 eV to strip a ground-state electron from a hydrogen atom,[49] compared to 2.23 million eV for splitting a deuterium nucleus.[50] Atoms are electrically neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called ions. Electrons that are farthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism, atoms are able to bond into molecules and other types of chemical compounds like ionic and covalent network crystals.[51]
>

>How atoms are constructed from electron orbitals and link to the periodic table
>
>>Link to GIF: https://en.wikipedia.org/wiki/File:Atomic_orbitals_and_periodic_table_construction.ogv
>>
>>Retrieved: 2 November 2017
>>Archive: https://archive.is/jyDBl
>>>
>>Electrons surround the nucleus in specific shapes, Atomic Orbitals.
>>>
>>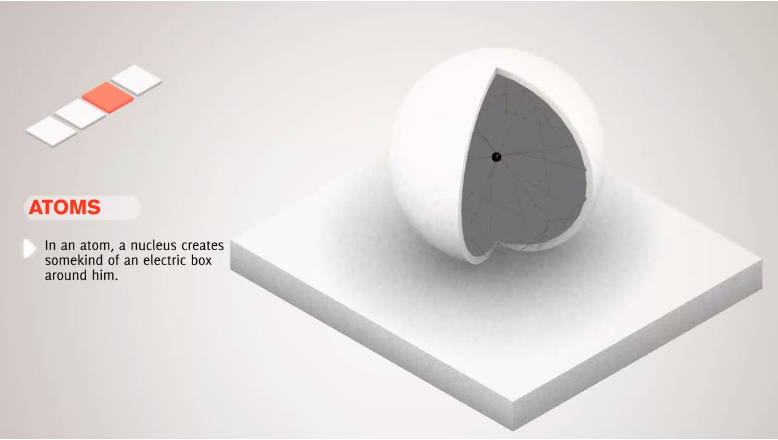
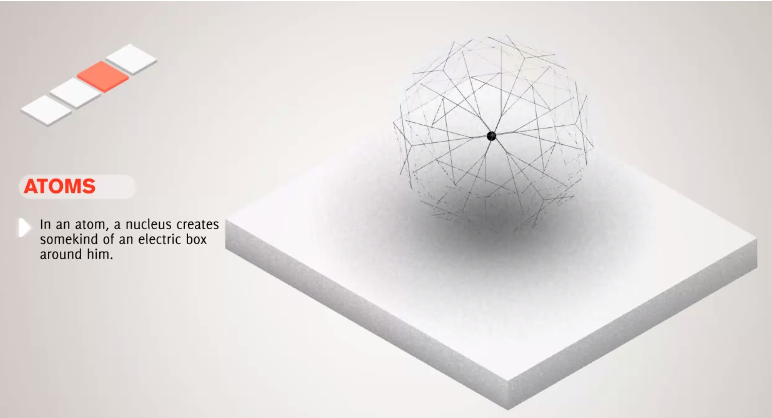
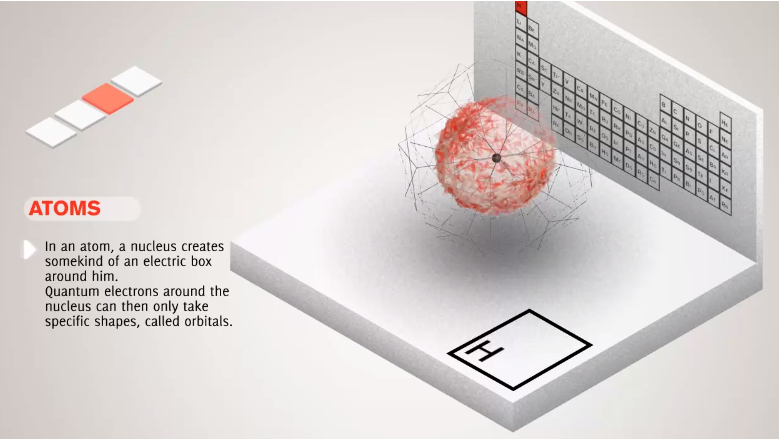
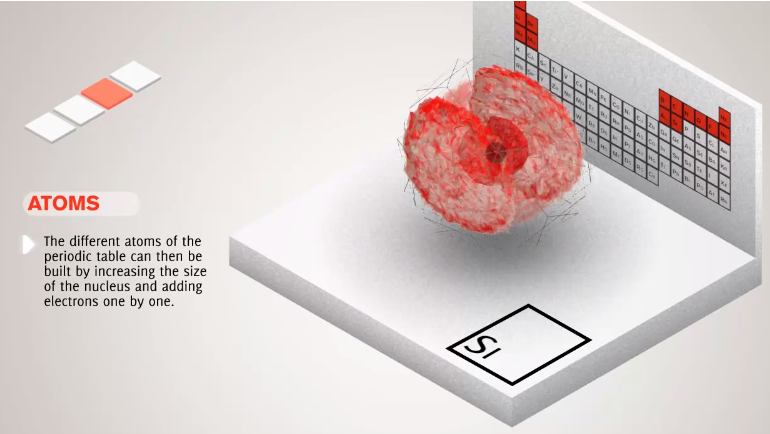
>
>Nuclear properties
>Main articles: Isotope, Stable isotope, List of nuclides, and List of elements by stability of isotopes
>
>By definition, any two atoms with an identical number of protons in their nuclei belong to the same chemical element. Atoms with equal numbers of protons but a different number of neutrons are different isotopes of the same element. For example, all hydrogen atoms admit exactly one proton, but isotopes exist with no neutrons (hydrogen-1, by far the most common form,[52] also called protium), one neutron (deuterium), two neutrons (tritium) and more than two neutrons. The known elements form a set of atomic numbers, from the single proton element hydrogen up to the 118-proton element oganesson.[53] All known isotopes of elements with atomic numbers greater than 82 are radioactive, although the radioactivity of element 83 (bismuth) is so slight as to be practically negligible.[54][55]
>
>…
>
>Mass
>Main articles: Atomic mass and mass number
>
>The large majority of an atom's mass comes from the protons and neutrons that make it up. The total number of these particles (called "nucleons") in a given atom is called the mass number. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. An example of use of a mass number is "carbon-12," which has 12 nucleons (six protons and six neutrons).
>
>The actual mass of an atom at rest is often expressed using the unified atomic mass unit (u), also called dalton (Da). This unit is defined as a twelfth of the mass of a free neutral atom of carbon-12, which is approximately 1.66×10<sup>-27</sup> kg.[59] Hydrogen-1 (the lightest isotope of hydrogen which is also the nuclide with the lowest mass) has an atomic weight of 1.007825 u.[60] The value of this number is called the atomic mass.
>
>…
>
>Shape and size
>Main article: Atomic radius
>
>Atoms lack a well-defined outer boundary, so their dimensions are usually described in terms of an atomic radius. This is a measure of the distance out to which the electron cloud extends from the nucleus.[63] However, this assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Atomic radii may be derived from the distances between two nuclei when the two atoms are joined in a chemical bond.
>
>The radius varies with the location of an atom on the atomic chart, the type of chemical bond, the number of neighboring atoms (coordination number) and a quantum mechanical property known as spin.[64] On the periodic table of the elements, atom size tends to increase when moving down columns, but decrease when moving across rows (left to right).[65] Consequently, the smallest atom is helium with a radius of 32 pm, while one of the largest is caesium at 225 pm.[66]
>
>When subjected to external forces, like electrical fields, the shape of an atom may deviate from spherical symmetry. The deformation depends on the field magnitude and the orbital type of outer shell electrons, as shown by group-theoretical considerations. Aspherical deviations might be elicited for instance in crystals, where large crystal-electrical fields may occur at low-symmetry lattice sites.[67][68] Significant ellipsoidal deformations have been shown to occur for sulfur ions[69] and chalcogen ions[70] in pyrite-type compounds.
>
>Atomic dimensions are thousands of times smaller than the wavelengths of light (400–700 nm) so they cannot be viewed using an optical microscope. However, individual atoms can be observed using a scanning tunneling microscope. To visualize the minuteness of the atom, consider that a typical human hair is about 1 million carbon atoms in width.[71] A single drop of water contains about 2 sextillion (2×10<sup>21</sup>) atoms of oxygen, and twice the number of hydrogen atoms.[72] A single carat diamond with a mass of 2×10<sup>-4</sup> kg contains about 10 sextillion (10<sup>22</sup>) atoms of carbon.[note 2] If an apple were magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple.[73]
>
>Radioactive decay
>Main article: Radioactive decay
>
>Every element has one or more isotopes that have unstable nuclei that are subject to radioactive decay, causing the nucleus to emit particles or electromagnetic radiation. Radioactivity can occur when the radius of a nucleus is large compared with the radius of the strong force, which only acts over distances on the order of 1 fm.[74]
>
>…
>
>Magnetic moment
>Main articles: Electron magnetic moment and Nuclear magnetic moment
>
>Elementary particles possess an intrinsic quantum mechanical property known as spin. This is analogous to the angular momentum of an object that is spinning around its center of mass, although strictly speaking these particles are believed to be point-like and cannot be said to be rotating. Spin is measured in units of the reduced Planck constant (h), with electrons, protons and neutrons all having spin ½ h, or "spin-½". In an atom, electrons in motion around the nucleus possess orbital angular momentum in addition to their spin, while the nucleus itself possesses angular momentum due to its nuclear spin.[77]
>
>>**MES Note:** Basically view these different spin/momentum properties as intrinsically energetic particles interacting with other intrinsically energetic particles, thus resulting in additional energetic properties.
>
>The magnetic field produced by an atom—its magnetic moment—is determined by these various forms of angular momentum, just as a rotating charged object classically produces a magnetic field. However, the most dominant contribution comes from electron spin. Due to the nature of electrons to obey the Pauli exclusion principle, in which no two electrons may be found in the same quantum state, bound electrons pair up with each other, with one member of each pair in a spin up state and the other in the opposite, spin down state. Thus these spins cancel each other out, reducing the total magnetic dipole moment to zero in some atoms with even number of electrons.[78]
https://en.wikipedia.org/wiki/Quantum_state
Retrieved: 3 November 2017
Archive: https://archive.is/QF1GX
Quantum state
>In quantum physics, quantum state refers to the state of an isolated quantum system. A quantum state provides a probability distribution for the value of each observable, i.e. for the outcome of each possible measurement on the system. Knowledge of the quantum state together with the rules for the system's evolution in time exhausts all that can be predicted about the system's behavior.
>
>
…
>
>For example, when dealing with the energy spectrum of the electron in a hydrogen atom, the relevant state vectors are identified by the principal quantum number n, the angular momentum quantum number l, the magnetic quantum number m, and the spin z-component sz.
https://en.wikipedia.org/wiki/Quantum_system
Retrieved: 3 November 2017
Archive: https://archive.is/VhSBI
Quantum system
>A quantum system is a portion of the whole Universe (environment or physical world) which is taken under consideration to make analysis or to study for quantum mechanics pertaining to the wave-particle duality in that system. Everything outside this system (i.e. environment) is studied only to observe its effects on the system. A quantum system involves the wave function and its constituents, such as the momentum and wavelength of the wave for which wave function is being defined.
**Back to the Atom Wikipedia Page:**
>In ferromagnetic elements such as iron, cobalt and nickel, an odd number of electrons leads to an unpaired electron and a net overall magnetic moment. The orbitals of neighboring atoms overlap and a lower energy state is achieved when the spins of unpaired electrons are aligned with each other, a spontaneous process known as an exchange interaction. When the magnetic moments of ferromagnetic atoms are lined up, the material can produce a measurable macroscopic field. Paramagnetic materials have atoms with magnetic moments that line up in random directions when no magnetic field is present, but the magnetic moments of the individual atoms line up in the presence of a field.[78][79]
>
>The nucleus of an atom will have no spin when it has even numbers of both neutrons and protons, but for other cases of odd numbers, the nucleus may have a spin. Normally nuclei with spin are aligned in random directions because of thermal equilibrium. However, for certain elements (such as xenon-129) it is possible to polarize a significant proportion of the nuclear spin states so that they are aligned in the same direction—a condition called hyperpolarization. This has important applications in magnetic resonance imaging.[80][81]
>
>Energy levels
>
>The potential energy of an electron in an atom is negative, its dependence of its position reaches the minimum (the most absolute value) inside the nucleus, and vanishes when the distance from the nucleus goes to infinity, roughly in an inverse proportion to the distance. In the quantum-mechanical model, a bound electron can only occupy a set of states centered on the nucleus, and each state corresponds to a specific energy level; see time-independent Schrödinger equation for theoretical explanation. An energy level can be measured by the amount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). The lowest energy state of a bound electron is called the ground state, i.e. stationary state, while an electron transition to a higher level results in an excited state.[82] The electron's energy raises when n increases because the (average) distance to the nucleus increases. Dependence of the energy on l is caused not by electrostatic potential of the nucleus, but by interaction between electrons.
https://en.wikipedia.org/wiki/Principal_quantum_number
Retrieved: 4 November 2017
Archive: https://archive.is/ZlzJo
Principle quantum number
>In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers which are assigned to each electron in an atom to describe that electron's state. As a discrete variable, the principal quantum number is always an integer. As n increases, the number of electronic shells increases and the electron spends more time farther from the nucleus. As n increases, the electron is also at a higher potential energy and is therefore less tightly bound to the nucleus.
>
>The principal quantum number was first created for use in the semiclassical Bohr model of the atom, distinguishing between different energy levels. With the development of modern quantum mechanics, the simple Bohr model was replaced with a more complex theory of atomic orbitals. However, modern theory still requires the principal quantum number. Apart from the principal quantum number, the other quantum numbers for bound electrons are the azimuthal quantum number, the magnetic quantum number, and the spin quantum number.
>
>For an analogy, one could imagine a multistoried building with an elevator structure. The building has an integer number of floors, and a (well-functioning) elevator which can only stop at a particular floor. Furthermore, the elevator can only travel an integer number of levels. As with the principal quantum number, higher numbers are associated with higher potential energy.
>
>Beyond this point the analogy breaks down; in the case of elevators the potential energy is gravitational but with the quantum number it is electromagnetic. The gains and losses in energy are approximate with the elevator, but precise with quantum state. The elevator ride from floor to floor is continuous whereas quantum transitions are discontinuous. Finally the constraints of elevator design are imposed by the requirements of architecture, but quantum behavior reflects fundamental laws of physics.
https://en.wikipedia.org/wiki/Azimuthal_quantum_number
Retrieved: 4 November 2017
Archive: https://archive.is/caeV4
Azimuthal quantum number
>The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital. The azimuthal quantum number is the second of a set of quantum numbers which describe the unique quantum state of an electron (the others being the principal quantum number, following spectroscopic notation, the magnetic quantum number, and the spin quantum number). It is also known as the orbital angular momentum quantum number, orbital quantum number or second quantum number, and is symbolized as l.
https://en.wikipedia.org/wiki/Atomic_orbital
Retrieved: 4 November 2017
Archive: https://archive.is/Td5sR
Atomic orbital
>In quantum mechanics, an atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom.[1] This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term, atomic orbital, may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.[2]
>
>Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, l, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number).
>
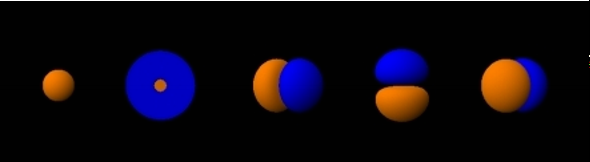
>The shapes of the first five atomic orbitals are: 1s, 2s, 2p<sub>x</sub>, 2p<sub>y</sub>, and 2p<sub>z</sub>. The two colors show the phase or sign of the wave function in each region. These are graphs of ?(x,y,z) functions which depend on the coordinates of one electron. To see the elongated shape of ?(x,y,z)<sup>2</sup> functions that show probability density more directly, see the graphs of d-orbitals below.
>
>>MES Note: I will cover the atomic orbitals in more detail in a later video so stay tuned!
**Back to the Atom Wikipedia Page:**
>For an electron to transition between two different states, e.g. grounded state to first excited level (ionization), it must absorb or emit a photon at an energy matching the difference in the potential energy of those levels, according to Niels Bohr model, what can be precisely calculated by the Schrödinger equation. Electrons jump between orbitals in a particle-like fashion. For example, if a single photon strikes the electrons, only a single electron changes states in response to the photon; see Electron properties.
>
>The energy of an emitted photon is proportional to its frequency, so these specific energy levels appear as distinct bands in the electromagnetic spectrum.[83] Each element has a characteristic spectrum that can depend on the nuclear charge, subshells filled by electrons, the electromagnetic interactions between the electrons and other factors.[84]
>
>When a continuous spectrum of energy is passed through a gas or plasma, some of the photons are absorbed by atoms, causing electrons to change their energy level. Those excited electrons that remain bound to their atom spontaneously emit this energy as a photon, traveling in a random direction, and so drop back to lower energy levels. Thus the atoms behave like a filter that forms a series of dark absorption bands in the energy output. (An observer viewing the atoms from a view that does not include the continuous spectrum in the background, instead sees a series of emission lines from the photons emitted by the atoms.) Spectroscopic measurements of the strength and width of atomic spectral lines allow the composition and physical properties of a substance to be determined.[85]
>
>An example of absorption lines in a spectrum
>
>Close examination of the spectral lines reveals that some display a fine structure splitting. This occurs because of spin–orbit coupling, which is an interaction between the spin and motion of the outermost electron.[86] When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the Zeeman effect. This is caused by the interaction of the magnetic field with the magnetic moment of the atom and its electrons. Some atoms can have multiple electron configurations with the same energy level, which thus appear as a single spectral line. The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines.[87] The presence of an external electric field can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the Stark effect.[88]
>
>If a bound electron is in an excited state, an interacting photon with the proper energy can cause stimulated emission of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon then move off in parallel and with matching phases. That is, the wave patterns of the two photons are synchronized. This physical property is used to make lasers, which can emit a coherent beam of light energy in a narrow frequency band.[89]
>
>Valence and bonding behavior
>Main articles: Valence (chemistry) and Chemical bond
>
>Valency is the combining power of an element. It is equal to number of hydrogen atoms that atom can combine or displace in forming compounds.[90] The outermost electron shell of an atom in its uncombined state is known as the valence shell, and the electrons in that shell are called valence electrons. The number of valence electrons determines the bonding behavior with other atoms. Atoms tend to chemically react with each other in a manner that fills (or empties) their outer valence shells.[91] For example, a transfer of a single electron between atoms is a useful approximation for bonds that form between atoms with one-electron more than a filled shell, and others that are one-electron short of a full shell, such as occurs in the compound sodium chloride and other chemical ionic salts. However, many elements display multiple valences, or tendencies to share differing numbers of electrons in different compounds. Thus, chemical bonding between these elements takes many forms of electron-sharing that are more than simple electron transfers. Examples include the element carbon and the organic compounds.[92]
>
>The chemical elements are often displayed in a periodic table that is laid out to display recurring chemical properties, and elements with the same number of valence electrons form a group that is aligned in the same column of the table. (The horizontal rows correspond to the filling of a quantum shell of electrons.) The elements at the far right of the table have their outer shell completely filled with electrons, which results in chemically inert elements known as the noble gases.[93][94]
>
>States
>Main articles: State of matter and Phase (matter)
>
>At temperatures close to absolute zero, atoms can form a Bose–Einstein condensate, at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale.[97][98] This super-cooled collection of atoms then behaves as a single super atom, which may allow fundamental checks of quantum mechanical behavior.[99]
>
>Snapshots illustrating the formation of a Bose–Einstein condensate
>
>Identification
>
>The scanning tunneling microscope is a device for viewing surfaces at the atomic level. It uses the quantum tunneling phenomenon, which allows particles to pass through a barrier that would normally be insurmountable. Electrons tunnel through the vacuum between two planar metal electrodes, on each of which is an adsorbed atom, providing a tunneling-current density that can be measured. Scanning one atom (taken as the tip) as it moves past the other (the sample) permits plotting of tip displacement versus lateral separation for a constant current. The calculation shows the extent to which scanning-tunneling-microscope images of an individual atom are visible. It confirms that for low bias, the microscope images the space-averaged dimensions of the electron orbitals across closely packed energy levels—the Fermi level local density of states.[100][101]
>
>Scanning tunneling microscope image showing the individual atoms making up this gold (100) surface. The surface atoms deviate from the bulk crystal structure and arrange in columns several atoms wide with pits between them (See surface reconstruction).
>
>An atom can be ionized by removing one of its electrons. The electric charge causes the trajectory of an atom to bend when it passes through a magnetic field. The radius by which the trajectory of a moving ion is turned by the magnetic field is determined by the mass of the atom. The mass spectrometer uses this principle to measure the mass-to-charge ratio of ions. If a sample contains multiple isotopes, the mass spectrometer can determine the proportion of each isotope in the sample by measuring the intensity of the different beams of ions.
>
>Origin and current state
>
>Atoms form about 4% of the total energy density of the observable Universe, with an average density of about 0.25 atoms/m<sup>3</sup>.[106] Within a galaxy such as the Milky Way, atoms have a much higher concentration, with the density of matter in the interstellar medium (ISM) ranging from 10<sup>5</sup> to 10<sup>9</sup> atoms/m<sup>3</sup>.[107]
>
>…
>
>Formation
>
>Electrons are thought to exist in the Universe since early stages of the Big Bang. Atomic nuclei forms in nucleosynthesis reactions. In about three minutes Big Bang nucleosynthesis produced most of the helium, lithium, and deuterium in the Universe, and perhaps some of the beryllium and boron.[111][112][113]
>
>…
https://en.wikipedia.org/wiki/Universe
Retrieved: 8 November 2017
Archive: https://archive.is/ylvRe
Universe
>The Universe is all of space and time (spacetime) and its contents,[12]which includes planets, moons, minor planets, stars, galaxies, the contents of intergalactic space and all matter and energy.[13][14] The size of the entire Universe is still unknown[6] with the latest figure, calculated by Halpern and Tomasello after the data from European Space Agency's Planck satellite estimating it to be 90.68 billion light-years across, 0.7% smaller then previously thought.[15]
>

>The Hubble Ultra-Deep Field image shows some of the most remote galaxies visible with present technology, each consisting of billions of stars. (Apparent image area about 1/79 that of a full moon)[1]
>
>>[https://commons.wikimedia.org/wiki/File:NASA-HS201427a-HubbleUltraDeepField2014-20140603.jpg](https://commons.wikimedia.org/wiki/File:NASA-HS201427a-HubbleUltraDeepField2014-20140603.jpg)
>>
>>Retrieved: 8 November 2017
>>Archive: https://archive.is/jSJu9
>>
>>>June 3, 2014
>>>
>>>RELEASE 14-151
>>>
>>>Hubble Team Unveils Most Colorful View of Universe Captured by Space Telescope
>>>
>>>http://www.nasa.gov/press/2014/june/hubble-team-unveils-most-colorful-view-of-universe-captured-by-space-telescope/ http://hubblesite.org/newscenter/archive/releases/2014/27
>>>
>>>Composite image showing the visible and near infrared light spectrum
>>>
>>>This is a composite image showing the visible and near infrared light spectrum collected from Hubble's ACS and WFC3 instruments over a nine-year period.
>
>…
>
>Constituent spatial scales of the observable universe
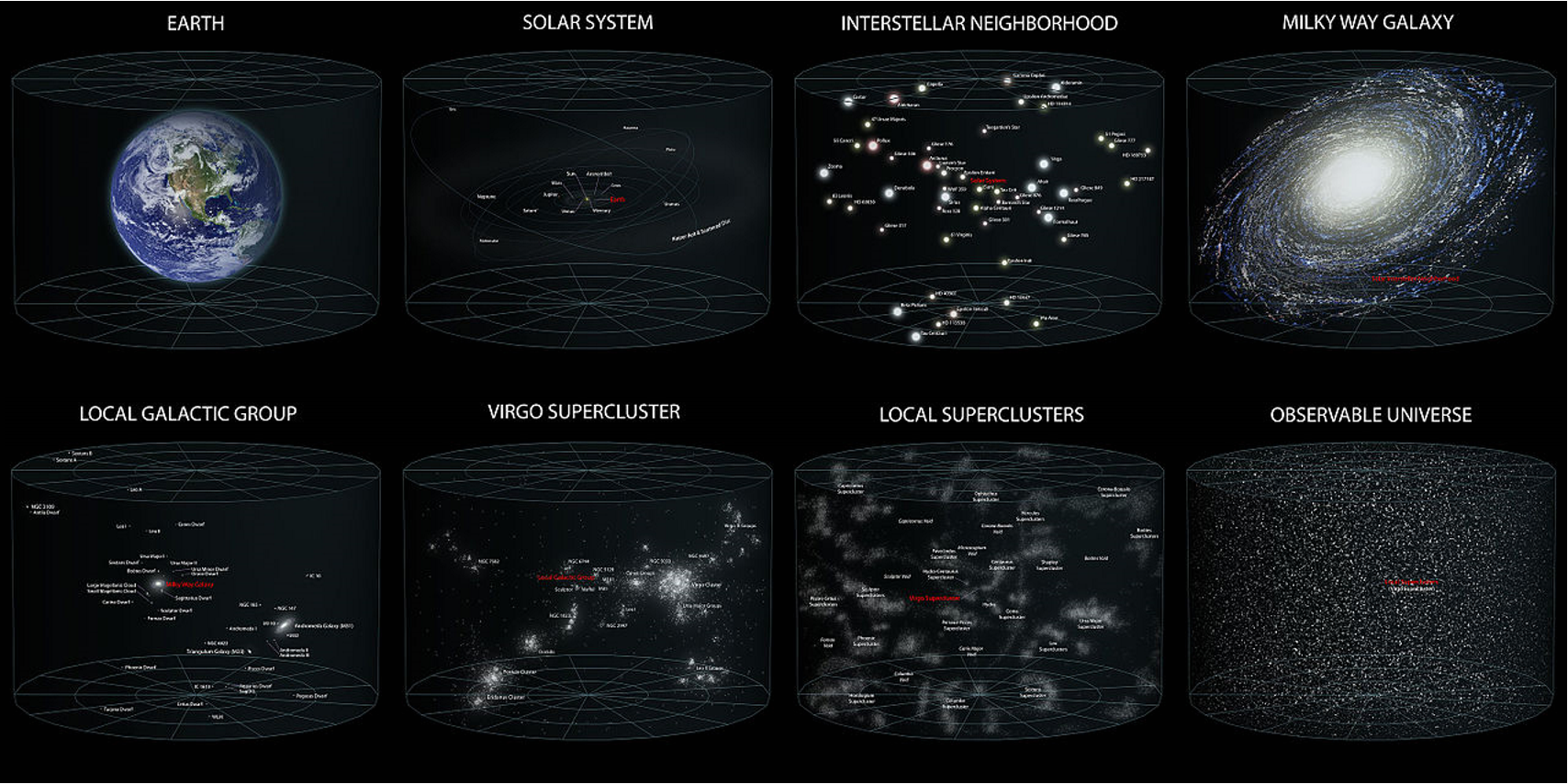
https://en.wikipedia.org/wiki/Observable_universe
Retrieved: 8 November 2017
Archive: https://archive.is/4Qgts
Observable universe
>The observable universe is a spherical region of the Universe comprising all matter that can be observed from Earth at the present time, because electromagnetic radiation from these objects have had time to reach Earth since the beginning of the cosmological expansion. There are at least 2 trillion galaxies in the observable universe,[7][8] containing more stars than all the grains of sand on planet Earth.[9][10][11]
>
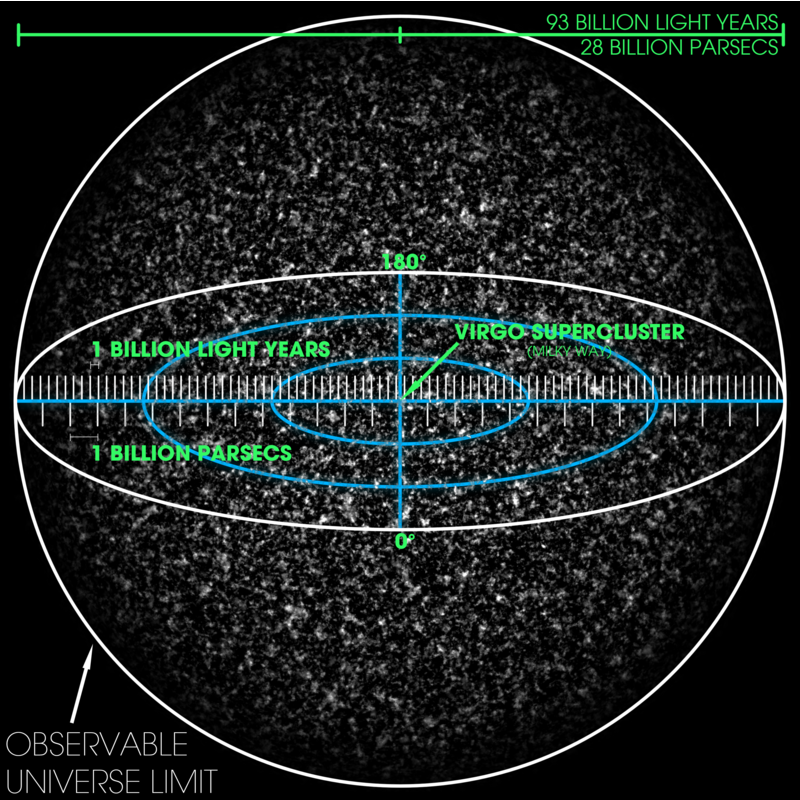
>Visualization of the whole observable universe. The scale is such that the fine grains represent collections of large numbers of superclusters. The Virgo Supercluster – home of Milky Way – is marked at the center, but is too small to be seen.
https://en.wikipedia.org/wiki/Galaxy
Retrieved: 8 November 2017
Archive: https://archive.is/sZ04l
Galaxy
>A galaxy is a gravitationally bound system of stars, stellar remnants, interstellar gas, dust, and dark matter.[1][2] The word galaxy is derived from the Greek galaxias (?a?a??a?), literally "milky", a reference to the Milky Way.
>

>NGC 4414, a typical spiral galaxy in the constellation Coma Berenices, is about 55,000 light-years in diameter and approximately 60 million light-years away from Earth.
https://en.wikipedia.org/wiki/Milky_Way
Retrieved: 6 November 2017
Archive: https://archive.is/6NoLq
Milky Way
>The Milky Way is the galaxy[21][22][23][nb 1] that contains our Solar System.[24]
>

>The descriptive "milky" is derived from the appearance from Earth of the galaxy – a band of light seen in the night sky formed from stars that cannot be individually distinguished by the naked eye.
https://en.wikipedia.org/wiki/Solar_System
Retrieved: 8 November 2017
Archive: https://archive.is/XQExa
Solar System
The Solar System[a] is the gravitationally bound system comprising the Sun and the objects that orbit it, either directly or indirectly.[b] Of those objects that orbit the Sun directly, the largest eight are the planets,[c] with the remainder being significantly smaller objects, such as dwarf planets and small Solar System bodies. Of the objects that orbit the Sun indirectly, the moons, two are larger than the smallest planet, Mercury.[d]
>The Sun and planets of the Solar System
>(distances not to scale)
>
>…
>

>The Solar System. Distances are to scale, objects are not.
https://en.wikipedia.org/wiki/Planet
Retrieved: 8 November 2017
Archive: https://archive.is/vlE4V
Planet
>A planet is an astronomical body orbiting a star or stellar remnant that
>
>• is massive enough to be rounded by its own gravity,
>• is not massive enough to cause thermonuclear fusion, and
>• has cleared its neighbouring region of planetesimals.[a][1][2]
>
>The term planet is ancient, with ties to history, astrology, science, mythology, and religion. Several planets in the Solar System can be seen with the naked eye. These were regarded by many early cultures as divine, or as emissaries of deities.
>

>The eight planets of the Solar System
>
>• The terrestrial planets: Mercury, Venus, Earth, and Mars
>
>• The giant planets: Jupiter and Saturn (gas giants), Uranus and Neptune (ice giants)
>
>Shown in order from the Sun and in true color. Sizes are not to scale.
https://en.wikipedia.org/wiki/Terrestrial_planet
Retrieved: 8 November 2017
Archive: https://archive.is/ORvdK
Terrestrial planet
>A terrestrial planet, telluric planet, or rocky planet is a planet that is composed primarily of silicate rocks or metals. Within the Solar System, the terrestrial planets are the inner planets closest to the Sun, i.e. Mercury, Venus, Earth, and Mars. The terms "terrestrial planet" and "telluric planet" are derived from Latin words for Earth (Terra and Tellus), as these planets are, in terms of structure, "Earth-like".
>
>Terrestrial planets have a solid planetary surface, making them substantially different from the larger giant planets, which are composed mostly of some combination of hydrogen, helium, and water existing in various physical states.
https://en.wikipedia.org/wiki/Planetesimal
Retrieved: 9 November 2017
Archive: https://archive.is/eFy8N
Planetesimal
>Planetesimals /plæn?'t?s?m?lz/ are solid objects thought to exist in protoplanetary disks and in debris disks.
>
>A widely accepted theory of planet formation, the so-called planetesimal hypotheses, the Chamberlin–Moulton planetesimal hypothesis and that of Viktor Safronov, states that planets form out of cosmic dust grains that collide and stick to form larger and larger bodies. When the bodies reach sizes of approximately one kilometer, then they can attract each other directly through their mutual gravity, enormously aiding further growth into moon-sized protoplanets.
https://en.wikipedia.org/wiki/Clearing_the_neighbourhood
Retrieved: 9 November 2017
Archive: https://archive.is/fpu48
Clearing the neighborhood
>"Clearing the neighbourhood around its orbit" is a criterion for a celestial body to be considered a planet in the Solar System. This was one of the three criteria adopted by the International Astronomical Union (IAU) in its 2006 definition of planet.[1] In 2015, a proposal was made to use the criterion in extending the definition to exoplanets.[2]
>
>In the end stages of planet formation, a planet (as so defined) will have "cleared the neighbourhood" of its own orbital zone, meaning it has become gravitationally dominant, and there are no other bodies of comparable size other than its satellites or those otherwise under its gravitational influence. A large body that meets the other criteria for a planet but has not cleared its neighbourhood is classified as a dwarf planet. This includes Pluto, which is constrained in its orbit by the gravity of Neptune and shares its orbital neighbourhood with Kuiper belt objects. The IAU's definition does not attach specific numbers or equations to this term, but all the planets have cleared their neighbourhoods to a much greater extent (by orders of magnitude) than any dwarf planet, or any candidate for dwarf planet.
https://en.wikipedia.org/wiki/Ceres_(dwarf_planet)
Retrieved: 9 November 2017
Archive: https://archive.is/sZRHf
Ceres (dwarf planet)
>Ceres (/'s??ri?z/;[19] minor-planet designation: 1 Ceres) is the largest object in the asteroid belt that lies between the orbits of Mars and Jupiter. Its diameter is approximately 945 kilometers (587 miles),[6] making it the largest of the minor planets within the orbit of Neptune. The 33rd-largest known body in the Solar System, it is the only dwarf planet within the orbit of Neptune.[c][20]Composed of rock and ice, Ceres is estimated to compose approximately one third of the mass of the entire asteroid belt. Ceres is the only object in the asteroid belt known to be rounded by its own gravity (though detailed analysis was required to exclude 4 Vesta). From Earth, the apparent magnitude of Ceres ranges from 6.7 to 9.3, and hence even at its brightest it is too dim to be seen with the naked eye except under extremely dark skies.
>
>Ceres was the first asteroid to be discovered (by Giuseppe Piazzi at Palermo on 1 January 1801). It was originally considered a planet, but was reclassified as an asteroid in the 1850s after many other objects in similar orbits were discovered.
>

>A view of Ceres in natural color, pictured by the Dawn spacecraft in May 2015.[a]
https://en.wikipedia.org/wiki/Asteroid_belt
Retrieved: 9 November 2017
Archive: https://archive.is/CnONu
Asteroid belt
>The asteroid belt is the circumstellar disc in the Solar System located roughly between the orbits of the planets Mars and Jupiter. It is occupied by numerous irregularly shaped bodies called asteroids or minor planets. The asteroid belt is also termed the main asteroid belt or main belt to distinguish it from other asteroid populations in the Solar System such as near-Earth asteroids and trojan asteroids.[1] About half the mass of the belt is contained in the four largest asteroids: Ceres, Vesta, Pallas, and Hygiea.[1] The total mass of the asteroid belt is approximately 4% that of the Moon, or 22% that of Pluto, and roughly twice that of Pluto's moon Charon (whose diameter is 1200 km).
>
>Ceres, the asteroid belt's only dwarf planet, is about 950 km in diameter, whereas 4 Vesta, 2 Pallas, and 10 Hygiea have mean diameters of less than 600 km.[2][3][4][5] The remaining bodies range down to the size of a dust particle.
>
>The asteroids of the inner Solar System and Jupiter: The donut-shaped asteroid belt is located between the orbits of Jupiter and Mars.

**MES Note:** Minor Planets and Dwarf Planets are not technically the same thing, but objects can be BOTH Minor Planets and Dwarf Planets.
http://rebrn.com/re/euler-diagram-of-solar-system-bodies-xpost-subcharts-229805/
Retrieved: 9 November 2017
Archive: https://archive.is/kqNoM
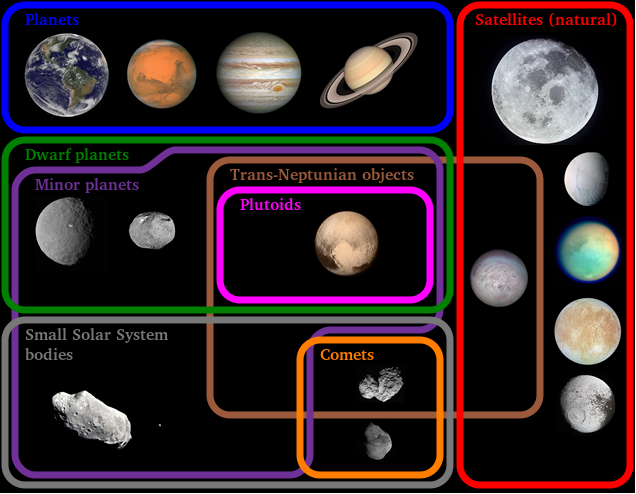
https://en.wikipedia.org/wiki/Pluto
Retrieved: 9 November 2017
Archive: https://archive.is/RHfqr
Pluto
>Pluto (minor-planet designation: 134340 Pluto) is a dwarf planet in the Kuiper belt, a ring of bodies beyond Neptune. It was the first Kuiper belt object to be discovered.
>
>Pluto was discovered by Clyde Tombaugh in 1930 and was originally considered to be the ninth planet from the Sun. After 1992, its status as a planet was questioned following the discovery of several objects of similar size in the Kuiper belt. In 2005, Eris, a dwarf planet in the scattered disc which is 27% more massive than Pluto, was discovered. This led the International Astronomical Union (IAU) to define the term "planet" formally in 2006, during their 26th General Assembly. That definition excluded Pluto and reclassified it as a dwarf planet.
>
>Pluto is the largest and second-most-massive known dwarf planet in the Solar System, and the ninth-largest and tenth-most-massive known object directly orbiting the Sun.

>Full-disc view of Pluto in near-true color, imaged by New Horizons[a]
**Back to the Atom Wikipedia Page:**
>Since the Big Bang, which produced no carbon or heavier elements, atomic nuclei have been combined in stars through the process of nuclear fusion to produce more of the element helium, and (via the triple alpha process) the sequence of elements from carbon up to iron;[115] see stellar nucleosynthesis for details.
>
>Isotopes such as lithium-6, as well as some beryllium and boron are generated in space through cosmic ray spallation.[116] This occurs when a high-energy proton strikes an atomic nucleus, causing large numbers of nucleons to be ejected.
>
>Elements heavier than iron were produced in supernovae through the r-process and in AGB stars through the s-process, both of which involve the capture of neutrons by atomic nuclei.[117] Elements such as lead formed largely through the radioactive decay of heavier elements.[118]
>
>>https://en.wikipedia.org/wiki/Supernova
>>
>>Retrieved: 8 November 2017
>>Archive: https://archive.is/T7dmn
>>
>>Supernova
>>
>>>A supernova (/?su?p?rno?v?/ plural: supernovae /?su?p?rno?vi?/ or supernovas, abbreviations: SN and SNe) is a transient astronomical event that occurs during the last stellar evolutionary stages of a massive star's life, whose dramatic and catastrophic destruction is marked by one final titanic explosion. This causes the sudden appearance of a "new" bright star, before slowly fading from sight over several weeks or months.

>>>SN 1994D (bright spot on the lower left), a Type Ia supernova out-shining its home galaxy NGC 4526
>
>Earth
>
>Most of the atoms that make up the Earth and its inhabitants were present in their current form in the nebula that collapsed out of a molecular cloud to form the Solar System. The rest are the result of radioactive decay, and their relative proportion can be used to determine the age of the Earth through radiometric dating.[119][120] Most of the helium in the crust of the Earth (about 99% of the helium from gas wells, as shown by its lower abundance of helium-3) is a product of alpha decay.[121]
>
>There are a few trace atoms on Earth that were not present at the beginning (i.e., not "primordial"), nor are results of radioactive decay. Carbon-14 is continuously generated by cosmic rays in the atmosphere.[122] Some atoms on Earth have been artificially generated either deliberately or as by-products of nuclear reactors or explosions.[123][124] Of the transuranic elements—those with atomic numbers greater than 92—only plutonium and neptunium occur naturally on Earth.[125][126] Transuranic elements have radioactive lifetimes shorter than the current age of the Earth[127] and thus identifiable quantities of these elements have long since decayed, with the exception of traces of plutonium-244 possibly deposited by cosmic dust.[128] Natural deposits of plutonium and neptunium are produced by neutron capture in uranium ore.[129]
>
>The Earth contains approximately 1.33×10<sup>50</sup> atoms.[130] Although small numbers of independent atoms of noble gases exist, such as argon, neon, and helium, 99% of the atmosphere is bound in the form of molecules, including carbon dioxide and diatomic oxygen and nitrogen. At the surface of the Earth, an overwhelming majority of atoms combine to form various compounds, including water, salt, silicates and oxides. Atoms can also combine to create materials that do not consist of discrete molecules, including crystals and liquid or solid metals.[131][132] This atomic matter forms networked arrangements that lack the particular type of small-scale interrupted order associated with molecular matter.[133]
>
>>https://en.wikipedia.org/wiki/Molecule
>>
>>Retrieved: 4 November 2017
>>Archive: https://archive.is/og3tx
>>
>>Molecule
>>
>>>A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.[4][5][6][7][8]
>>>
>>>…
>>>
>>>The definition of the molecule has evolved as knowledge of the structure of molecules has increased. Earlier definitions were less precise, defining molecules as the smallest particles of pure chemical substances that still retain their composition and chemical properties.[13] This definition often breaks down since many substances in ordinary experience, such as rocks, salts, and metals, are composed of large crystalline networks of chemically bonded atoms or ions, but are not made of discrete molecules.
>>
>>Thus the sentence *"Atoms can also combine to create materials that do not consist of discrete molecules, including crystals and liquid or solid metals"* implies that combination/bonding of atoms (or ions) can form materials that aren't molecules by their technical definition, i.e. "discrete" or distinct molecules.
>
>Rare and theoretical forms
>
>Superheavy elements
>Main article: Transuranium element
>
>While isotopes with atomic numbers higher than lead (82) are known to be radioactive, an "island of stability" has been proposed for some elements with atomic numbers above 103. These superheavy elements may have a nucleus that is relatively stable against radioactive decay.[134] The most likely candidate for a stable superheavy atom, unbihexium, has 126 protons and 184 neutrons.[135]
>
>Exotic matter
>Main article: Exotic matter
>
>Each particle of matter has a corresponding antimatter particle with the opposite electrical charge. Thus, the positron is a positively charged antielectron and the antiproton is a negatively charged equivalent of a proton. When a matter and corresponding antimatter particle meet, they annihilate each other. Because of this, along with an imbalance between the number of matter and antimatter particles, the latter are rare in the universe. The first causes of this imbalance are not yet fully understood, although theories of baryogenesis may offer an explanation. As a result, no antimatter atoms have been discovered in nature.[136][137] However, in 1996 the antimatter counterpart of the hydrogen atom (antihydrogen) was synthesized at the CERN laboratory in Geneva.[138][139]
>
>Other exotic atoms have been created by replacing one of the protons, neutrons or electrons with other particles that have the same charge. For example, an electron can be replaced by a more massive muon, forming a muonic atom. These types of atoms can be used to test the fundamental predictions of physics.[140][141][142]
**MES Note:** For as much as I have been bashing Wikipedia for being the Orwellian Perception Management tool that it is, this particular article is actually very thorough and understandable, albeit only the mainstream science narrative, especially for such a complex topic such as the atom!
https://en.wikipedia.org/wiki/Muon
Retrieved: 4 November 2017
Archive: https://archive.is/lIK64
Muon
>The muon (/'mju??n/; from the Greek letter mu (µ) used to represent it) is an elementary particle similar to the electron, with an electric charge of -1 e and a spin of 1/2, but with a much greater mass. It is classified as a lepton. As is the case with other leptons, the muon is not believed to have any sub-structure — that is, it is not thought to be composed of any simpler particles.
>
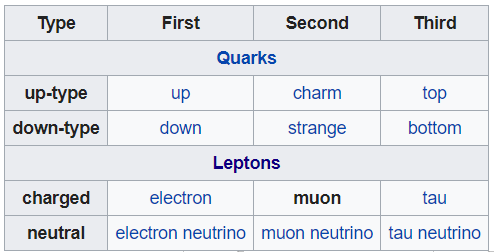
>Generations of matter
>
>…
>
>Muonic heavy hydrogen atoms with a negative muon may undergo nuclear fusion in the process of muon-catalyzed fusion, after the muon may leave the new atom to induce fusion in another hydrogen molecule. This process continues until the negative muon is trapped by a helium atom, and cannot leave until it decays.
https://en.wikipedia.org/wiki/Quark
Retrieved: 4 November 2017
Archive: https://archive.is/pKzOm
Quark
>A quark (/'kw??rk/ or /'kw??rk/) is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nuclei.[1] Due to a phenomenon known as color confinement, quarks are never directly observed or found in isolation; they can be found only within hadrons, such as baryons (of which protons and neutrons are examples) and mesons.[2][3] For this reason, much of what is known about quarks has been drawn from observations of the hadrons themselves.
>
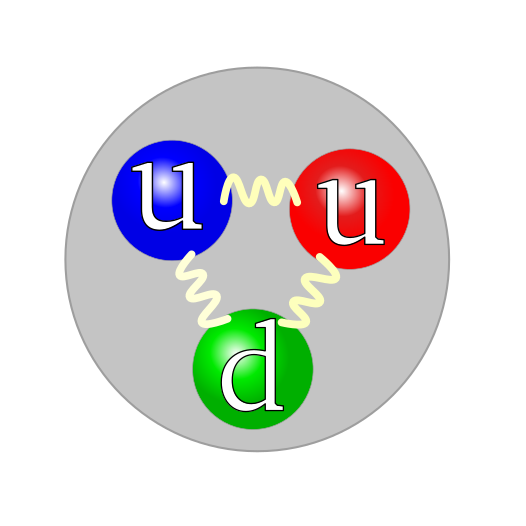
>A proton is composed of two up quarks, one down quark, and the gluons that mediate the forces "binding" them together. The color assignment of individual quarks is arbitrary, but all three colors must be present.
https://en.wikipedia.org/wiki/Lepton
Retrieved: 4 November 2017
Archive: https://archive.is/nmwIC
Lepton
>A lepton is an elementary, half-integer spin (spin 1/2) particle that does not undergo strong interactions.[1] Two main classes of leptons exist: charged leptons (also known as the electron-like leptons), and neutral leptons (better known as neutrinos). Charged leptons can combine with other particles to form various composite particles such as atoms and positronium, while neutrinos rarely interact with anything, and are consequently rarely observed. The best known of all leptons is the electron.
>
>There are six types of leptons, known as flavours, forming three generations.[2] The first generation is the electronic leptons, comprising the electron (e<sup>-</sup>) and electron neutrino (v<sub>e</sub>); the second is the muonic leptons, comprising the muon (µ<sup>-</sup>) and muon neutrino (v<sub>µ</sub>); and the third is the tauonic leptons, comprising the tau (t<sup>-</sup>) and the tau neutrino (v<sub>t</sub><sup>-</sup>). Electrons have the least mass of all the charged leptons. The heavier muons and taus will rapidly change into electrons and neutrinos through a process of particle decay: the transformation from a higher mass state to a lower mass state. Thus electrons are stable and the most common charged lepton in the universe, whereas muons and taus can only be produced in high energy collisions (such as those involving cosmic rays and those carried out in particle accelerators).
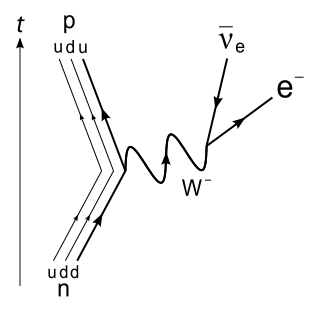
>Leptons are involved in several processes such as beta decay.
>
>…
>
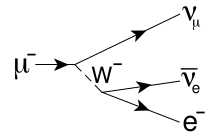
>A muon transmutes into a muon neutrino by emitting a W<sup>-</sup> boson. The W<sup>-</sup> boson subsequently decays into an electron and an electron antineutrino.
**Recall from #FreeEnergy Part 2:**
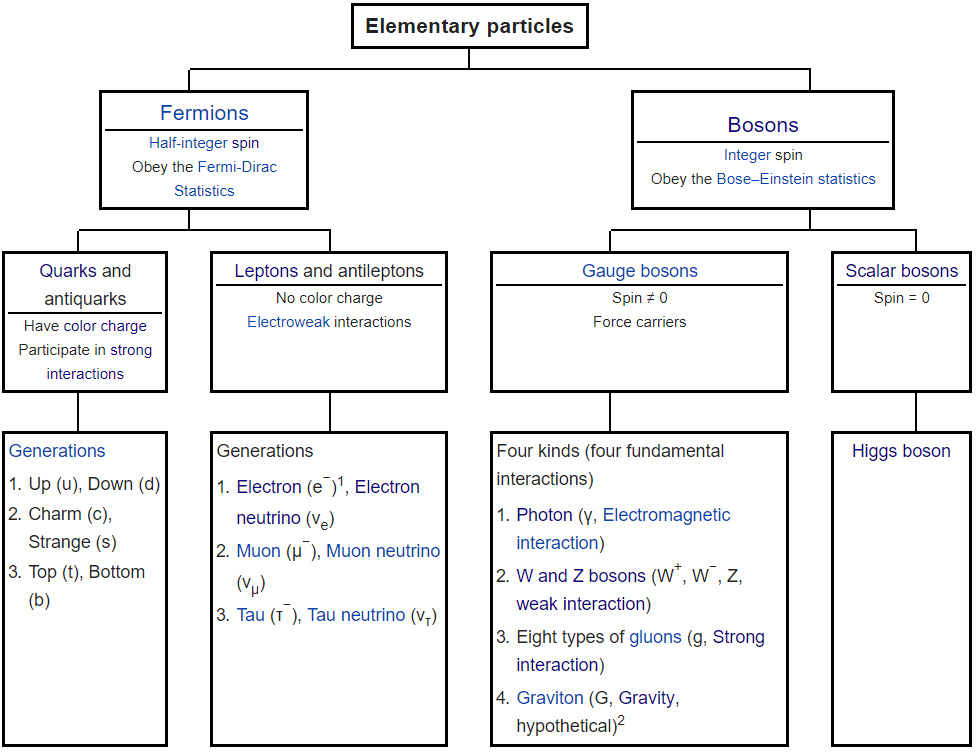
https://en.wikipedia.org/wiki/W_and_Z_bosons
Retrieved: 4 November 2017
Archive: https://archive.is/Rfr2b
W and Z bosons
>The W and Z bosons are together known as the weak or more generally as the intermediate vector bosons. These elementary particles mediate the weak interaction; the respective symbols are W<sup>+</sup>, W<sup>-</sup>, and Z. The W bosons have either a positive or negative electric charge of 1 elementary charge and are each other's antiparticles. The Z boson is electrically neutral and is its own antiparticle. The three particles have a spin of 1. The W bosons have a magnetic moment, but the Z has none. All three of these particles are very short-lived, with a half-life of about 3×10<sup>-25</sup> s. Their experimental discovery was a triumph for what is now known as the Standard Model of particle physics.
>
>>https://en.wikipedia.org/wiki/Truly_neutral_particle
>>
>>Retrieved: 8 November 2017
>>Archive: https://archive.is/dk6J1
>>
>>Truly neutral particle
>>
>>>In particle physics, a truly neutral particle is a subatomic particle with all its charges equal to zero. This not only requires particles to be electrically neutral, but also requires that all of their other charges (like the colour charge) are neutral. Such a particle will be its own antiparticle.
>>>
>>>Mathematically, charge conjugation replaces all the constituent particles of a particle with their corresponding antiparticles. If a particle remains the same after charge conjugation, then it is its own antiparticle, and is truly neutral.
>>>
>>>Known examples of such elementary particles include photons, Z bosons, and Higgs bosons, along with the hypothetical neutralinos, sterile neutrinos, and gravitons. For a spin-1/2 particle such as the neutralino, being a truly neutral particle implies being a Majorana fermion.
>>>
>>>Composite particles can also be truly neutral. The best known example is onium, a system composed of a particle forming a bound state with its own antiparticle.
>
>The W bosons are named after the weak force. The physicist Steven Weinberg named the additional particle the "Z particle",[3] and later gave the explanation that it was the last additional particle needed by the model. The W bosons had already been named, and the Z bosons have zero electric charge.[4]
>
>The two W bosons are verified mediators of neutrino absorption and emission. During these processes, the W boson charge induces electron or positron emission or absorption, thus causing nuclear transmutation. The Z boson is not involved in the absorption or emission of electrons and positrons.
>
>The Z boson mediates the transfer of momentum, spin and energy when neutrinos scatter elastically from matter (a process which conserves charge). Such behavior is almost as common as inelastic neutrino interactions and may be observed in bubble chambers upon irradiation with neutrino beams. Whenever an electron is observed as a new free particle suddenly moving with kinetic energy, it is inferred to be a result of a neutrino interacting directly with the electron, since this behavior happens more often when the neutrino beam is present. In this process, the neutrino simply strikes the electron and then scatters away from it, transferring some of the neutrino's momentum to the electron. Because neutrinos are neither affected by the strong force nor the electromagnetic force, and because the gravitational force between subatomic particles is negligible, such an interaction can only happen via the weak force. Since such an electron is not created from a nucleon, and is unchanged except for the new force impulse imparted by the neutrino, this weak force interaction between the neutrino and the electron must be mediated by an electromagnetically neutral, weak-force boson particle. Thus, this interaction requires a Z boson.
>
>Basic properties
>
>These bosons are among the heavyweights of the elementary particles. With masses of 80.4 GeV/c<sup>2</sup> and 91.2 GeV/c<sup>2</sup>, respectively, the W and Z bosons are almost 100 times as massive as the proton – heavier, even, than entire iron atoms. Their high masses limit the range of the weak interaction. By way of contrast, the photon is the force carrier of the electromagnetic force and has zero mass, consistent with the infinite range of electromagnetism; the hypothetical graviton is also expected to have zero mass. (Although gluons are also presumed to have zero mass, the range of the color force is limited for different reasons; see color confinement.)
>
>…

>The Feynman diagram for beta decay of a neutron into a proton, electron, and electron antineutrino via an intermediate heavy W boson
https://www.antonine-education.co.uk/Pages/Physics_1/Particles/PP11/particles_page_11.htm
Retrieved: 4 November 2017
Archive: https://archive.is/VRiWW
Particle Physics Tutorial 11 - Particle Interactions
>…
>
>However, there are some further rules as suggested by the Institute of Physics:
>
>• A Feynman diagram represents before, during, and after.
>• The interaction is shown by a line going upwards at a diagonal. You can only go forwards in time.
>• A wiggly line represents an interaction made by a photon.

>• A straight dotted line represents a W- or W+ boson. You don’t need to know about a Z.

>• A “curly-wurly” line represents a gluon.

>• The arrow from a particle is away from the interaction; the arrow for the antiparticle points towards the interaction.
>
>So the Feynman diagram for out beta decay becomes:
>
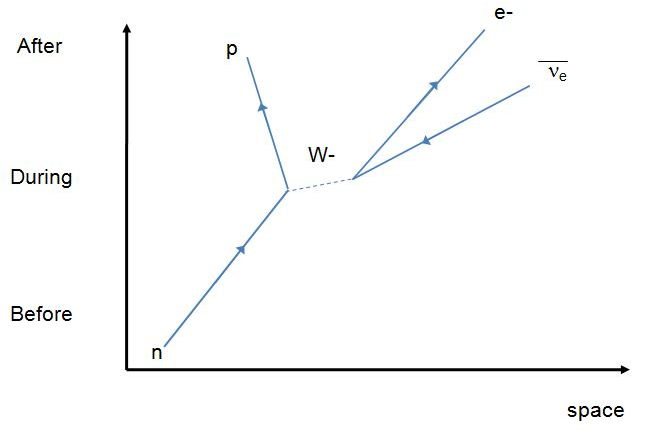
https://en.wikipedia.org/wiki/Feynman_diagram
Retrieved: 4 November 2017
Archive: https://archive.is/JtQCf
Feynman diagram
>In theoretical physics, Feynman diagrams are pictorial representations of the mathematical expressions describing the behavior of subatomic particles. The scheme is named after its inventor, American physicist Richard Feynman, and was first introduced in 1948. The interaction of sub-atomic particles can be complex and difficult to understand intuitively. Feynman diagrams give a simple visualization of what would otherwise be a rather arcane and abstract formula. As David Kaiser writes, "since the middle of the 20th century, theoretical physicists have increasingly turned to this tool to help them undertake critical calculations", and so "Feynman diagrams have revolutionized nearly every aspect of theoretical physics".[1] While the diagrams are applied primarily to quantum field theory, they can also be used in other fields, such as solid-state theory.
>
>Feynman used Ernst Stueckelberg's interpretation of the positron as if it were an electron moving backward in time.[2] Thus, antiparticles are represented as moving backward along the time axis in Feynman diagrams.
>
>
>In this Feynman diagram, an electron and a positron annihilate, producing a photon (represented by the blue sine wave) that becomes a quark–antiquark pair, after which the antiquark radiates a gluon (represented by the green helix).
>
>…
>
>• space x and time t axes are not always shown, directions of external lines correspond to passage of time.
>
>…
>
>Feynman diagram of electron/positron annihilation
**MES Note:** Important to keep in mind the space/time axes aren't always shown and aren't always in the same orientation, so keep the context in mind when reading them.
https://en.wikipedia.org/wiki/Muon-catalyzed_fusion
Retrieved: 4 November 2017
Archive: https://archive.is/E2eYd
Muon-catalyzed fusion
>Muon-catalyzed fusion (µCF) is a process allowing nuclear fusion to take place at temperatures significantly lower than the temperatures required for thermonuclear fusion, even at room temperature or lower. It is one of the few known ways of catalyzing nuclear fusion reactions.
>
>Muons are unstable subatomic particles. They are similar to electrons, but are about 207 times more massive. If a muon replaces one of the electrons in a hydrogen molecule, the nuclei are consequently drawn 196[1][2] times closer than in a normal molecule, due to the reduced mass being 196 times the mass of an electron. When the nuclei are this close together, the probability of nuclear fusion is greatly increased, to the point where a significant number of fusion events can happen at room temperature.
>
>Current techniques for creating large numbers of muons require large amounts of energy, larger than the amounts produced by the catalyzed nuclear fusion reactions. This prevents it from becoming a practical power source. Moreover, each muon has about a 1% chance of "sticking" to the alpha particle produced by the nuclear fusion of a deuteron with a triton, removing the "stuck" muon from the catalytic cycle, meaning that each muon can only catalyze at most a few hundred deuterium tritium nuclear fusion reactions. So, these two factors, of muons being too expensive to make and then sticking too easily to alpha particles, limit muon-catalyzed fusion to a laboratory curiosity. To create useful room-temperature muon-catalyzed fusion, reactors would need a cheaper, more efficient muon source and/or a way for each individual muon to catalyze many more fusion reactions.
>
>…
>
>More recent measurements seem to point to more encouraging values for the a-sticking probability, finding the a-sticking probability to be about 0.5% (or perhaps even about 0.4% or 0.3%), which could mean as many as about 200 (or perhaps even about 250 or about 333) muon-catalyzed d-t fusions per muon.[8][note 4] Indeed, the team led by Steven E. Jones achieved 150 d-t fusions per muon (average) at the Los Alamos Meson Physics Facility.[9] Unfortunately, 200 (or 250 or even 333) muon-catalyzed d-t fusions per muon is still not enough to reach break-even. Even with break-even, the conversion efficiency from thermal energy to electrical energy is only about 40% or so, further limiting viability. The best recent estimates of the electrical "energy cost" per muon[note 5] is about 6 GeV with accelerators that are (coincidentally) about 40% efficient at transforming electrical energy from the power grid into acceleration of the deuterons.
>
>As of 2012, no practical method of producing energy through this means has been published, although some discoveries using the Hall effect show promise.[10][not in citation given]
**INDEED** that is the same Steven E. Jones behind the coverup of BOTH Cold Fusion and 9/11!!!
See #FreeEnergy Part 2 and #911Truth Part 6 to learn more about this pure evil creature.
[https://youtu.be/g091Z8xaDqU](https://youtu.be/g091Z8xaDqU)
Retrieved: 4 November 2017
Archive: https://archive.is/EYmua
This is a great video to quickly get an overview of how Muon Catalysed Fusion works.
The resulting muonic hydrogen atom draws another hydrogen atom together and the heavy muon compresses the nucleons together until they fuse.
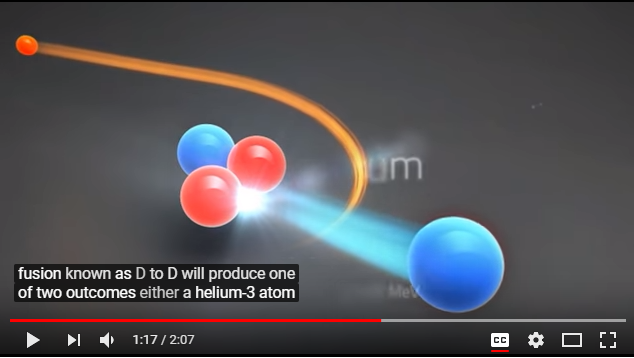
When a neutron and muon get ejected, a helium-3 atom is formed releasing energy. The ejected muon can then repeat the process with other hydrogen atoms.
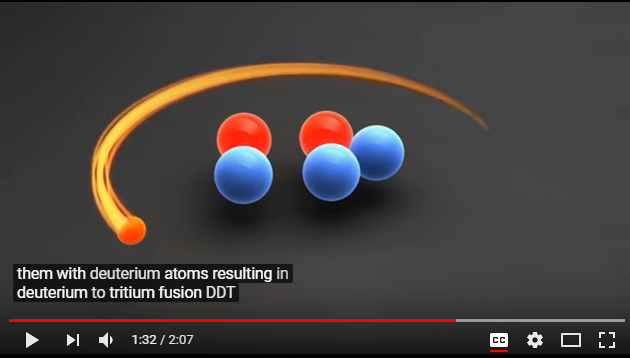
The best scenario is when a muonic deuterium atom fuses with a tritium atom (DT Fusion) resulting in a more stable helium-4 atom, and more energy released, than DD Fusion.
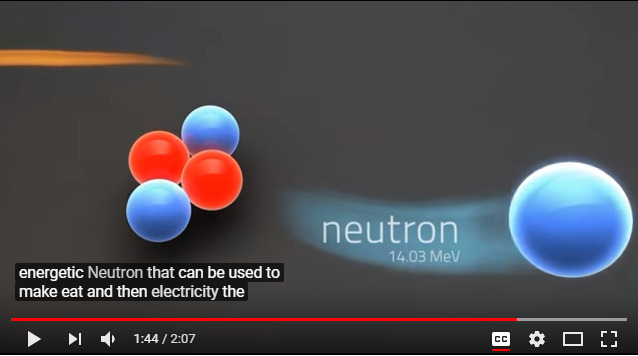
Very interesting stuff, and even more interesting Steven Jones connection in Muon Catalysed Fusion, the coverup of Cold Fusion, and the Controlled Opposition of 9/11 #FreeEnergy Technology! #LockHimUp
**Summary**
A good way for me to learn quickly is to teach others, read out loud, and then summarize the main points in point form. This may seem tedious, and it is, but as I have shown in many of my videos, the gold nuggets of bombshell information is usually hidden in the finer details. And unlike most media outlets, science outlets, and online content producers I built upon the findings of each video I make. Thus I HAVE to get each point as right as possible, because anything other than the absolute truth, such as 9/11, could lead to disastrous consequences.
Thus, let's dive into a summary of the mainstream science background of the atomic structure:
**Atoms, Matter, Nucleus, Protons, Electrons, Ions, Isotopes**
- An atom is the smallest unit of ordinary matter that has the properties of a chemical element.
- Chemical elements constitute all of the ordinary matter in the universe.
- Ordinary matter makes up 15% of the matter in the universe.
- Dark matter constitutes the remainder unknown matter in the universe, and is not made of chemical elements.
- Atoms are small enough to undergo quantum effects.
- Atoms comprise of a nucleus with one or more electrons bound to it.
- Nucleus is made up of protons and neutrons, called nucleons.
- Nucleus accounts for 99.94% of the atom's mass.
- A Chemical Element is defined by the number of protons inside the nucleus of an atom.
- The number of protons in the nucleus defines the chemical element the atom belongs to.
- The number of neutrons in the nucleus defines the isotope of the chemical element.
- The number of electrons influences the magnetic properties of the atom.
- Protons have a positive electric charge, +.
- Neutrons have zero electric charge.
- Electrons have a negative electric charge, -.
- An electrically neutral atom has the same number of protons and electrons.
- An ion is an atom, or molecule, with a different number of protons and electrons, thus has a net overall charge.
- Elements often times react in ratios of small whole numbers.
- The Atomic Mass Number is the total number of protons and neutrons in a nucleus because these nucleons account for a large majority of the atom's mass.
- Atoms are thousands of times smaller than the wavelengths of visible light.
- Atoms form about 4% of the total energy density of the observable universe.
- The earth contains 1.33x10<sup>50</sup> atoms.
- Hydrogen is the lightest Atom but not the smallest atom, Helium is. #VeryInteresting
- If an apple were magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple. #WOW
**Chemical Bonds, Chemical Compounds, Ionic Bonds, Covalent Bonds**
- Atoms can attach to one or more atoms by chemical bonds to form chemical compounds such as molecules.
- Molecules are electrically neutral groups of two or more atoms held together by chemical bonds.
- Chemical bonds are lasting attractions between atoms, ions, or molecules that enables formation of chemical compounds.
- Ionic Bonds are chemical bonds arising from the electrostatic force of attraction between oppositely charged ions.
- Covalent Bonds are chemical bonds involving the sharing of electrons.
**Electromagnetic Force, Nuclear Force, Nuclear Transmutation**
- Electrons are attracted to the protons by the Electromagnetic Force.
- Protons and neutrons in the nucleus are attracted to each other by the Nuclear Force.
- The nuclear force is usually stronger than the electromagnetic force repelling the positively charged protons from each other.
- When the electromagnetic force becomes stronger than the nuclear force, nucleons can be ejected, thus forming a different element; hence Nuclear Decay resulting in Nuclear Transmutation.
**Electrical Conductors, Holes, Electrodes, Circuits**
- An Electrical Conductor is a material that allows the flow of an electrical current in one or more directions.
- Electrical current is generated by the flow of negatively charged electrons, positively charged holes or +/- ions.
- Holes, or Electron Holes, are locations with the absence of electrons which otherwise should be located, leaving a net positive charge.
- Holes are imaginary particles used instead of describing the resulting equivalent movement of many separate electrons due to the presence of holes.
- Electrodes are electrical conductors used to make contact with nonmetallic parts of an electrical circuit.
- An Electronic Circuit is a combination of electrical components interconnected by conductors through which electric current flows.
**Polarity, Electric Polarity, Ground, Voltage, Vacuum**
- Polarity is an attribute with two possible values.
- An electric charge can have either positive or negative polarity.
- A magnet has a polarity: "north" and "south" poles.
- Electrical Polarity is the direction of current flow; positive or negative.
- Current flows from the positive pole (+) to the negative pole (-), i.e. by convention in the direction of a positive charge.
- Electrons flow from the negative pole to the positive pole, i.e. opposite to the current.
- Electrical Ground is the reference point for voltage measurements or a return path for electric currents.
- Electrical Ground can be a direct physical connection to the Earth.
- Electrical Ground has applications in ensuring electrical safety such as limiting build-up of static electricity.
- Voltage is the difference in electric potential between two points per unit electric charge.
- Voltage is also referred to as electrical potential difference, electric pressure, or electric tension.
- The symbols for voltage is formally ?V or ?U but sometimes just V or U.
- Electric Potential, also called electric field potential or electrostatic potential, is the amount of work needed to move a unit positive charge from a reference point to a specific point inside the field without producing any acceleration.
- A perfect Vacuum is space without any matter.
**Cathode Rays, Vacuum Tubes, Thermionic Emission, Filament, Photoelectric Effect**
- Cathode rays are streams of electrons observed in vacuum tubes.
- Cathode rays are also called electron beams or e-beams.
- A Vacuum Tube is a device that controls electric current between electrodes in an evacuated container.
- Vacuum Tubes are also referred to as Electron Tubes or just Tubes.
- Similar Tubes are gas-filled instead of evacuated.
- Electron Tubes can involve Thermionic Emission or Photoelectric Emission.
- Thermionic Emission is the heat induced flow of electric charge resulting from the thermal energy overcoming the material's work function.
- The Work Function is the minimum energy needed to remove an electron from a solid so that it is far from the surface on the atomic scale but close enough not to be influenced by ambient electric fields.
- The Work Function is a characteristic of the surface of the material, i.e. crystal face and contamination) and not of the bulk material itself.
- Filament is conducting wire or thread with a high melting point.
- The Photoelectric Effect is the emission of electrons (or other charge carriers) when light is shone onto a material.
- Quantum Mechanics, by describing light as quantized or discrete photons, better describes the Photoelectric Effect than does classical physics.
- Electrons are only emitted when the frequency/energy of the light exceeds the material's threshold frequency.
- Intensity (or amount of light) or duration of light exposure increases the amount of electrons being emitted but ONLY if the threshold frequency is exceeded.
- No electrons will be emitted if the threshold frequency is not exceeded regardless of the amount/intensity or duration of light shone on the material; in contrast to expected results from classical physics.
**Thermal Radiation, Incandescence, Fluorescence, Electroluminescence, Black Body Thermal Radiation, Fluorescence**
- Thermal Radiation is electromagnetic radiation generated by the kinetic motion of charged particles in all matter with greater temperature than absolute zero, due to inter-atomic collisions which causes atoms or molecules to change, i.e. in charge-acceleration and/or dipole oscillations.
- Incandescence is the emission of electromagnetic radiation, such as visible light, from a hot body due to its temperature; Incandescence often refers to just emission of visible light whereas Thermal Radiation is the general emission of any electromagnetic radiation
- An Incandescent Light Bulb involves heating filament so that it glows.
- Most solid and liquid substances start to glow at about 798 K (525 °C) (977 °F) and glow from red to white in increasing temperature.
- Electroluminescence is when material emits light in response to the passage of an electric current or to an electric field and is the result of the recombination of electrons and electron holes thus releasing energy in the form of photons.
- Light-Emitting Diodes (LED) is a two-lead semiconductor light source utilizing a p-n junction and is an application of Electroluminescence.
- Car's instrument panel and speedometer lighting are applications of Electroluminescence.
- A lead is a conductive electrical connection consisting of a length of wire or metal that comes from a device.
- A p-n junction is the interface between two types of semiconductor material, p-type and n-type.
- p-type or positive side has an excess of electron holes.
- n-side has an excess of electrons.
- A Black Body is an idealized physical body of matter that absorbs all electromagnetic radiation directed at it.
- A White Body reflects Electromagnetic Radiation.
- Black Body Radiation is the thermal electromagnetic radiation within or around a body in thermodynamic equilibrium with its environment, or emitted by a black body, and is its spectrum and intensity is simplified as being depended only on the body's temperature.
- All normal matter emits electromagnetic radiation when it has a temperature above absolute zero and represents a conversion of the body's thermal energy into electromagnetic energy, hence called Thermal Radiation.
- The human body radiates energy as Infrared Light.
- Thermodynamic Equilibrium is when no macroscopic energy or matter enter or exit a closed system.
- Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation.
- Most cases the emitted light has a longer wavelength (lower energy and lower frequency) than the light absorbed.
- When Ultra Violet, invisible to the naked eye, is absorbed the emitted light is visible to the naked eye, thus producing striking and distinct colors.
- Fluorescence involves an electron going from a higher energy level to a lower one and emitting a photon.
**Semiconductors, Insulators, Doping, Transistors, Superconductors**
- Semiconductors are materials with electrical conductivity between conductors such as copper and insulators such as glass.
- Semiconductors can have their properties altered by introducing impurities into them, and is known as doping.
- Intrinsic semiconductors have no significant dopant added, and are also called undoped or i-type semiconductors.
- Insulators are materials whose internal electric charges don't move freely and thus very little electric current will flow through it when in an electric field.
- Glass, paper, rubber, and plastics are good examples of good insulators.
- Insulating materials are employed for electrical wiring and cables.
- Transistors are semiconductor devices used to amplify or switch electronic signals/current/power.
- Transistors work by using the current it receives to modify the current it is connected to.
- Transistors' ability to be mass produced and their endless applications make it one of the greatest inventions ever, and is currently used in almost all modern electronics.
- Superconductors have exactly zero electrical resistance and completely eject magnetic field lines from its interior when cooled below a critical temperature.
- Conductivity increases as temperature drops.
- The ejection of magnetic field lines, known as the Meissner Effect, indicates Superconductors can't be understood as simply "Perfect Conductors" according to classical physics.
**Condensed Matter Physics, Solid State Physics**
- Condensed Matter Physics deals with the physical properties of condensed phases of matter, where the particles adhere to each other such as in solids and liquids.
- Solid State Physics is the study of rigid matter or solids and is the largest branch of condensed matter physics.
- Solid State Physics studies how large scale properties of solid materials result from their atomic-scale properties.
- The way atoms are packed, either in regular patterns such as metals and ice or irregularly like in glass, greatly affects the properties of the solid.
- Crystals or Crystalline Solids are solids made up of regularly packed atoms and comprise the bulk of Solid State Physics.
**Alpha Particles, Radionuclides, Ionizing Radiation**
- Alpha Particles, a, consist of two protons and two neutrons bound together and are identical to a helium nucleus, <sup>4</sup><sub>2</sub>He<sup>2+</sup>.
- Alpha Particles arise mainly from Alpha Decay in which a nucleus ejects Alpha Particles, hence referred to as Alpha Radiation
- The use of the term Alpha Particle or Helium are interchangeable.
- Radionuclides (or radioactive nuclide, radioisotope or radioactive isotopes) are atoms that have excess nuclear energy, making them unstable and can emit this energy has gamma radiation, alpha decay, or beta decay, or transfer this excess energy to one of its electrons.
- Ionization radiation is radiation that carries enough energy to liberate electrons from atoms or molecules, thus ionizing them.
- Beta Decay involves the conversion of a neutron to an electron or positron (antielectron) and being ejected out of the nucleus.
- Ionization Radiation is made up of energetic subatomic particles, ions, or atoms moving at high speeds, and electromagnetic waves at the upper part of the UV spectrum and higher.
**Hydrogen Isotopes, Protium, Deuterium, Tritium, Hydrons, Hydrides**
- Hydrogen is a chemical element characterized by having 1 proton.
- Naturally occurring Isotopes of Hydrogen include: Protium, Deuterium, and Tritium.
- Protium has zero neutrons, and is the most common isotope of hydrogen and is often just referred to as Hydrogen in normal context.
- Deuterium has 1 neutron.
- Tritium has 2 neutrons.
- A Hydron is any Hydrogen isotope with zero electrons.
- A Protium Hydron is a Proton, <sup>1</sup>H<sup>+</sup>. (Note: The symbol P is already taken by Phosphorus).
- A Deuterium Hydron is a Deuteron, <sup>2</sup>H<sup>+</sup> or D<sup>+</sup>.
- A Tritium Hydron is a Triton, <sup>3</sup>H<sup>+</sup> or T<sup>+</sup>.
- A Hydride is the anion of Hydrogen and has two electrons, H<sup>-</sup>.
**Bohr Model, Electron Cloud, Atomic Orbitals, Energy Levels, Quantum States & Numbers**
- The Bohr Model is an obsolete model describing how electrons of an atom were assumed to orbit the nucleus in a finite set of orbits and could jump between orbits through discrete changes of energy.
- Electrons are attracted to the protons in the nucleus by the electromagnetic force which binds them inside an electrostatic potential well.
- The potential well requires an external source of energy to allow the electron to escape.
- The electron cloud is a 3-dimensional region inside the potential well which is defined by an Atomic Orbital.
- The Atomic Orbital is a mathematical function characterizing the probability that an electron appears at a particular location in the Electron Cloud.
- Atomic Orbitals exist as discrete or quantized possible wave patterns and differ from each other in size, shape, and orientation.
- Each Atomic Orbital corresponds to a particular energy level of the electron.
- Quantum Mechanical Systems or Particles that are bound (i.e. confined spatially) can only take on certain discrete energy values, called Energy Levels; in contrast to classical physics.
- Energy Levels correspond to specific Quantum states.
- Quantum States refer to the probability distribution for the value of each observable or measurable outcome in a Quantum System, such as energy required to bind an electron in its orbital, the angular momentum, the magnetic moment, and the spin.
- Quantum Numbers are discrete numbers given to describe the values of Quantum States.
**Chemical Bonding, Covalent Bonds, Electronegativity, Ionic Bonding**
- Chemical bonds between atoms involve the interactions between their constituent electrons.
- A Covalent Bond, or Molecular Bond, is a chemical bond that involves the sharing of electrons between atoms, often in electron pairs.
- Covalent Bonding requires atoms to be of comparable or similar electronegativity.
- Electronegativity is the property that describes an atom's tendency to attract electrons towards itself.
- An Ionic Bond is a chemical bond composed of ions held together by electrostatic forces.
**Ionic Compounds, Van Der Waals Forces, Intermolecular & Intramolecular Forces**
- Ionic Compounds are neutral overall but made up of positively and negatively charged ions.
- Individual ions in ionic compounds are part of a continuous three-dimensional network, usually in a crystalline structure.
- Acids are ionic compounds containing hydrogen ions, H<sup>+</sup>.
- Bases are ionic compounds containing hydroxide, OH<sup>-</sup>, or oxide, O<sup>2-</sup>.
- Salts are ionic compounds without Acid/Base ions and can be formed by Acid-Base Reactions.
- Ionic compounds have high melting and boiling points and are hard and brittle.
- Ionic Compounds are almost always electrically insulating but become highly conductive when melted or dissolved because the ions are mobilized.
- Ionic Compounds are rarely purely ionic (i.e. held together only by electrostatic forces) and usually have some covalent interactions as well, as well as Van der Waals Interactions.
- Ionic Compounds also have a small additional attractive force from Van der Waals interactions.
- Van der Waals Forces are distance-dependent interactions between atoms or molecules and can be attractive or repulsive, and are due to uneven distribution of molecular charges, i.e. electric dipoles, either temporary or permanently.
- An Electric Dipole is a separation of positive or negative charges.
- Van der Waals include London Dispersion, Keesom, and Debye Forces.
- London Dispersion Forces are forces between instantaneously generated dipoles due to the random uneven distribution of electrons in an atom or molecule.
- Keesom Forces are forces between permanent molecular dipoles, known as Polar Molecules.
- Polar Molecules are molecules made up of atoms with dissimilar electronegativities create permanent dipoles.
- Debye Forces are forces between Polar Molecules and Molecules with induced polarity from Polar Molecules.
- Van der Waals Forces part of the group of Intermolecular Forces (IMFs) which are forces between molecules and other types of particles.
- Intermolecular Forces are weak relative to the forces that hold molecules together, Intramolecular Forces.
- Hydrogen Bonds can be either intramolecular or intermolecular, and is an electrostatic attraction between a Hydrogen atom covalently bound to a highly electronegative atom which experiences the electrostatic field of another highly electronegative atom nearby.
**Subatomic Particles, Nuclear Fusion/Fission, Spin, Quantum State**
- Protons and Neutrons are composite particles composed of elementary particles called quarks.
- Electrons are elementary particles.
- The number of protons and neutrons in the atomic nucleus can be modified, resulting in Nuclear Fusion when combined to formed a heavier nucleus, and Nuclear Fission when a nucleus is split into smaller nuclei.
- Spin is an intrinsic property of elementary particles similar to angular momentum but are point-like.
- Electrons in motion around the nucleus possess orbital angular momentum in addition to their spin.
- The nucleus itself possess angular momentum due to its spin.
- An Atom's magnetic moment is the magnetic field due to the above various forms of angular momentum, and mainly from electron spin, which cancel each other in some atoms with even number of electrons.
- In some materials, such as ferromagnetic elements, the magnetic moments of their atoms line up to produce a measurable macroscopic magnetic field.
- The nucleus of an atom will have no spin when it has an even number of both neutrons and protons, but may have a spin for cases of odd numbers.
**Atomic Spectral/Emission Lines, Absorption Bands, Valency**
- When an electron absorbs or emits enough photon energy it can jump to orbitals with higher or lower energy levels, respectively.
- The electrons that absorb photons but remain bound to the atom, are periodically excited due to the increase in energy level but then they spontaneously emit this photon energy in a random direction to drop back its energy level.
- The absorption of photon energy can be seen as Dark Absorption Bands when a continuous spectrum of energy is passed through a gas or plasma.
- To the observer away from the input spectrum of energy, the photon energy released by the excited electrons are seen as Atomic Spectral or Emission Lines.
- Valency is the combining power of an element and is equal to the number of hydrogen atoms that atom can combine or displace in forming compounds.
- The outermost electron shell of an atom in its uncombined state is known as the valence shell and determines the bonding behavior with other atoms.
- Chemically inert elements have their outer shells completely filled with electrons and are known as noble gases.
**Bose-Einstein Condensate, Quantum Tunneling, Rare and Theoretical Atoms, Exotic Atoms**
- The Bose-Einstein Condensate is a state of matter in which a collection of atoms super-cooled to near absolute zero behave as a single super atom thus experiencing quantum mechanical effects at macroscopic scale! #WOW
- Quantum Tunneling involves quantum particles moving past a barrier classically insurmountable.
- Scanning Tunneling Microscopes can measure atomic structures by inducing an electrical current as electrons tunnel through the vacuum between two electrodes (each of which contain an adsorbed atom) as the tip of one electrode gets close enough to the atoms to allow for Quantum Tunneling to occur.
- Isotopes with atomic numbers higher than Lead (82) are known to be radioactive, but a theoretical "Island of Stability" may exist in which Superheavy Elements can remain stable.
- Antimatter are extremely rare and have not been discovered in nature, even though each particle of matter has a corresponding antimatter particle.
- Exotic atoms have been created by replacing one of the protons, neutrons, or electrons with other particles of the same charge.
- Replacing an electron with a more massive muon forms a muonic atom.
**Universe, Observable Universe, Galaxy, Milky Way, Solar System, Planets, Dwarf Planets, Asteroid Belt, Ceres Dwarf Planet, Pluto, Supernovas**
- The Universe is of unknown size and is all of space and time (spacetime) which include all matter and energy.
- The Observable Universe is a spherical region of the Universe that can be observed from Earth at the present time.
- A Galaxy is a gravitationally bound system of stars, gas, dust, stellar remnants, and dark matter.
- The Milky Way is the galaxy that contains our Solar System.
- The Solar System is a gravitationally bound system comprising the Sun and objects that orbit it.
- Our Solar System has 8 planets orbiting around the Sun.
- Planets are astronomical bodies orbiting a star (or stellar remnant) that are massive enough to be rounded by its own gravity but not massive enough to cause thermonuclear fusion, and whose gravity has "Cleared the Neighborhood" of any objects in its orbit.
- Dwarf Planets meet some requirements of being a planet but have not cleared its neighborhood of surrounding objects in its orbit.
- **MES Note:** Minor Planets vs. Dwarf Planets are very badly defined in my opinion, and are not the same thing but objects can be both Minor and Dwarf Planets… Let me know if you can wrap your head around it!
- The 4 closest planets from the Sun are the tiny Earth-like planets: Mercury, Venus, Earth, Mars.
- The 4 farthest planets from the Sun are the giant planets: Jupiter and Saturn (Gas giants), and Uranus and Neptune (Ice Giants)
- MES Note: Order of the 8 planets are in increasing distant from the Sun.
- The Asteroid Belt is a disc of asteroids, rocks, and matter orbiting a vast area between the orbits of Mars and Jupiter.
- Ceres is the largest object in the Asteroid Belt, the only one large enough in the belt to have its shape rounded due to gravity, but has not become gravitationally dominant to separate from the Astroid Belt thus is considered a Dwarf Planet.
- Pluto was originally thought to be the 9th and farthest planet, but later definitions and discovery of similar sized objects in or near its orbit has changed its classification to a Dwarf Planet.
- Supernovas (or supernovae) involves the final titanic explosion of a massive star appearing as a "new" bright star momentarily before disappearing into darkness over several weeks or months.
**Muons, Quarks, Leptons, Particle Decay, W and Z Bosons**
- Muons, µ, are elementary particles similar to electrons, with the same electric charge, but with a much greater mass.
- A Muon is a Lepton.
- Leptons are elementary particles that don't undergo strong interactions.
- Leptons are classed as Charged (or electron-like) and Neutral (or neutrinos).
- Charged Leptons combine to form composite particles such as atoms, while neutrinos rarely interact with anything, thus are rarely observed.
- Quarks are elementary particles that do undergo strong interactions to combine to create composite particles such as protons and neutrons.
- Muons are unstable and rapidly change into electrons and neutrinos, through a process of Particle Decay.
- Muons transmute into a muon neutrino by emitting a W<sup>-</sup> Boson which subsequently decays into an electron and electron antineutrino (the antiparticle or antimatter of electron neutrino).
- W and Z Bosons are elementary particles that mediate the Weak Nuclear Interaction in Nuclear Transmutation.
- W and Z Bosons are 100 times as massive as the proton, and heavier than even entire iron atoms, but their heavy mass limits the range of the Weak Interaction.
- W<sup>-</sup> and W<sup>+</sup> bosons are negatively and positively electrically charged, respectively, and are each other's antiparticle.
- The Z boson is electrically neutral, its own antiparticle, and mediates the transfer of momentum, spin, and energy when neutrinos scatter elastically (i.e. conserve energy) from matter.
- Truly Neutral Particles are subatomic particles with all its charges equal to zero, not just electric charges, and including color charges.
**Feynman Diagrams, Muon-Catalyzed Fusion, Disinfo Agent Steven E. Jones….**
- Feynman Diagrams describe the behavior of subatomic particles through space and time.
-- Antiparticles are represented as moving backward along the time axis, hence point towards the interaction.
-- Wiggly Lines, i.e. sine waves, represent photons.
-- Straight Dotted Lines represent W bosons.
-- Helixes represent gluons.
- Muon-Catalyzed Fusion, µCF, is the process of by which replacing a Hydrogen's electron with the much heavier muon particle results in a much tighter orbit that can induce nuclear fusion by other nearby hydrogen particles, at low or normal temperatures just as with Cold Fusion.
- The desired outcome of µCF is the fusion of Deuterium with Tritium (DT) forming Helium-4, which releases the muon to repeat the process.
- The difficulty in production of muons and high probability of muons "sticking" to helium atoms, thus getting trapped and decaying thus ending the fusion process, make µCF inefficient and impractical.
- As also explained in #FreeEnergy Part 2 and in #911Truth Part 6, disinfo Agent Steven E. Jones was heavily involved in µCF research while leading a team of researchers at Los Almos National Laboratory in New Mexico, USA, (same place where the Atomic Bomb was created) and used µCF to coverup the viability of Pons & Fleischman's potentially ground-breaking Cold Fusion research!
- This is the same Steven E. Jones behind the freemason logo-bearing Architects & Engineers for 9/11 "Truth" pushing the bogus "Thermite" nonsense to coverup Dr. Judy Wood's brilliant work proving the existence and usage of Directed #FreeEnergy Technology on 9/11 to turn 7 buildings into dust before our very eyes!
This was another extensive review into Nuclear Physics, and as always Disinfo Agent Steven E. Jones has to make an appearance hahaha #YouCantMakeThisStuffUp
Anyways, if you tuned in this far in this video, I hope this has opened your understanding of Mainstream Science!
I will be covering more Mainstream Science in later parts in hopes of developing a firm understanding of any possible background information needed in my pursuit of #FreeEnergy Technology.
And as always, I put an extreme amount of time into my research so please consider donating even just a dollar to my Patreon or any one time donations through PayPal, Steemit, Bitcoin, or other Cryptocurrencies! https://mes.fm/donate
### Stay tuned for #FreeEnergy Part 4…👍 mes, steemiteducation, hotrix, primerz, blocktrader, ausbitbot, azizbd, schoolforsdg4, shammi, dreamarif, steemitbd, dokter-purnama, alol, drdave, saimdang, fataelrumy, ashfia, hanshotfirst, steemwizards, lordvader, calypso, brahma, steemship, beerbloke, candyman, teacherspet, curie, ratticus, anwenbaumeister, hendrikdegrote, rayken04, kushed, pharesim, sethlinson, shahzadnisar, sieses, toxichan, steemedia, cebymaster, pacokam8, mitthradiumn, wandereronwheels, bp423, diggerdugg, justdentist, bitopia, blackwidow7, bitrocker2020, tanyaschutte, robins, kevinwong, cotidiana, awesomianist, kouloumos, ovij, bcrafts, smartonelegal, newsandviews, dahbou, yosif, partitura.stem,